(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting held in Washington, DC between September 29th and October 2nd, 2024, was host to a prostate cancer treatment intensification session. Dr. Martin King presented the long-term results of the 44/20/0 trials that prospectively evaluated supplemental external beam radiotherapy in higher-risk prostate cancer patients implanted with Pd-103.
Low dose rate (LDR) brachytherapy is a curative treatment modality for prostate cancer and has demonstrated excellent long-term oncologic outcomes in both the monotherapy and boost settings. The selection of monotherapy versus boost regimens remains controversial, particularly for patients with intermediate-risk disease.
RTOG 0232 was a phase III trial of 588 patients with cT1c-T2bN0M0 prostate cancer with either Gleason Score ≥7 disease or PSA 10-20 ng/ml, who were randomized to either:
- External beam radiotherapy (EBRT; 45 Gy in 25 fractions) to the prostate + seminal vesicles + brachytherapy boost (110 Gy if 125-Iodine, 100 Gy if 103-Pd)
- Brachytherapy boost
The combination of EBRT + brachytherapy boost did not improve 5-year freedom from progression. Furthermore, this combination was associated with increased toxicity.1
However, to date, brachytherapy monotherapy is not recommended by the NCCN guidelines for patients with unfavorable intermediate-risk disease. As such, Dr. King argued that additional prospective data are needed in this space.
RTOG 44/20 and 20/0 were two serial randomized controlled trials, which evaluated whether incremental decreases in supplemental EBRT doses, in conjunction with increases in brachytherapy doses, impacted biochemical failure outcomes. In his presentation, Dr. King presented the long-term results of each of these trials. Additionally, he presented the results of analyses evaluating whether the biological equivalent dose was associated with biochemical failure in this combined cohort, as well as the individual subgroups of favorable- and unfavorable intermediate-risk disease.
The trial schema for RTOG 44/20 and 20/0 are summarized below:
From a technical standpoint, the pre-plan was as follows:
- Prostate target volume: prostate + 5 mm margin, except 0 mm margin posteriorly and 10 mm proximal seminal vesicle.
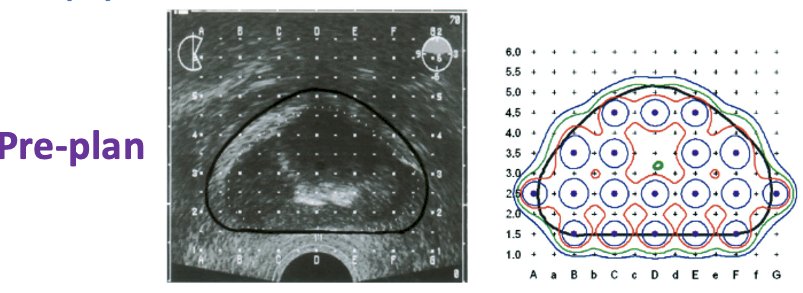
Intra-operative optimization and day 0 CT dosimetry were both performed.
For EBRT, the clinical target volume (CTV) was the prostate gland and seminal vesicles. The prostate target volume included a 2 cm margin, except for 1 cm posteriorly. Androgen deprivation therapy (ADT) was administered at the treating physician’s discretion. Follow-up (serum PSA levels and survival status) was performed every 3 to 6 months. Biochemical failure was defined as PSA >0.4 ng/ml. Patients were considered to have died secondary to prostate cancer if they experienced death with evidence of castration resistance or metastatic disease was present.
The total biologic equivalent dose (BED) was calculated utilizing the AAPM TG-137 formulation.
For each trial, univariable Fine and Gray modeling was used to evaluate the associations between the treatment received and biochemical failure/prostate cancer-specific mortality. In the combined trials, both univariable and multivariable Fine and Gray analyses evaluated the associations between BED and biochemical failure/prostate cancer-specific mortality.
The baseline patient characteristics for the RTOG 44/20 trial are summarized below. This trial included 247 patients who were randomized to either 44 Gy EBRT (n=125) or 20 Gy EBRT (n=122). 58% and 16% of patients had unfavorable intermediate- and high-risk disease, respectively. More patients in the 44 Gy arm received ADT (37% versus 27%).
The baseline patient characteristics for the RTOG 20/0 trial are summarized below. This trial included 383 patients who were randomized to either 20 Gy EBRT (n=196) or 0 Gy EBRT (n=187). 53% and 46% of patients had favorable and unfavorable intermediate-risk disease, respectively. More patients in the 20 Gy EBRT arm received ADT (10% versus 6%).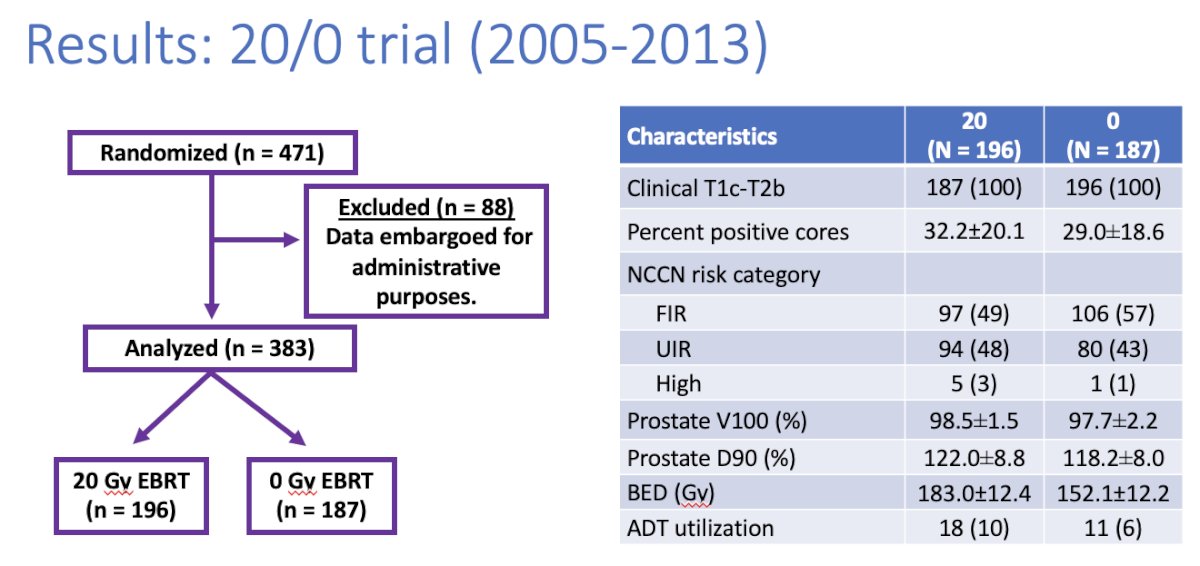
There were no differences in biochemical failure rates across the treatment arms in either trial:

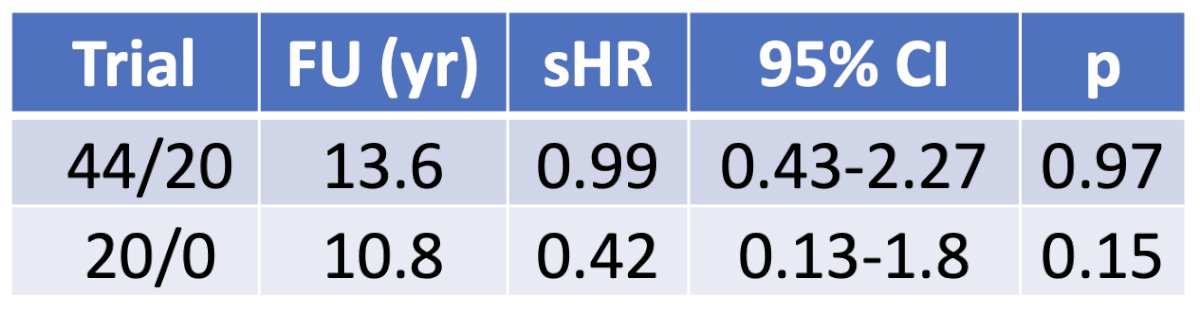
Similarly, there were no differences in prostate cancer-specific mortality between the treatment arms in either trial. Notably, there were no prostate cancer deaths in the RTOG 20/0 trial.
On multivariable analysis, a higher risk group (high or unfavorable intermediate versus favorable intermediate) was associated with increased rates of biochemical failure, whereas a more recent year of treatment was associated with decreased rates of biochemical failure. Notably, the BED was not associated with the rate of biochemical failure on either univariable or multivariable analysis.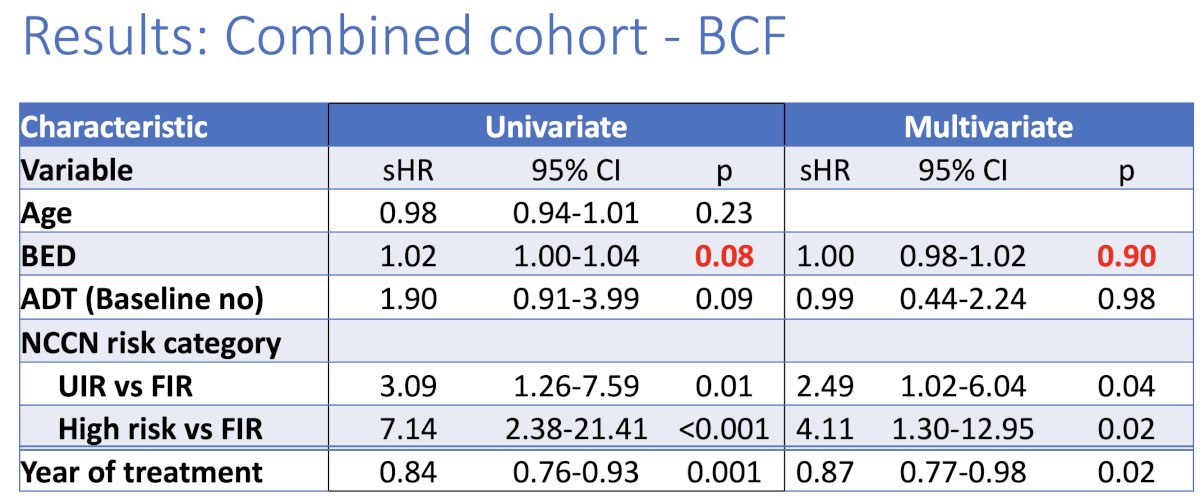
With respect to prostate cancer-specific mortality, BED was significantly associated with prostate cancer-specific mortality rates on univariable, but not multivariable analysis.
When analysis was stratified by the risk group (favorable and unfavorable intermediate risk), there were similarly no differences in biochemical failure survival outcomes.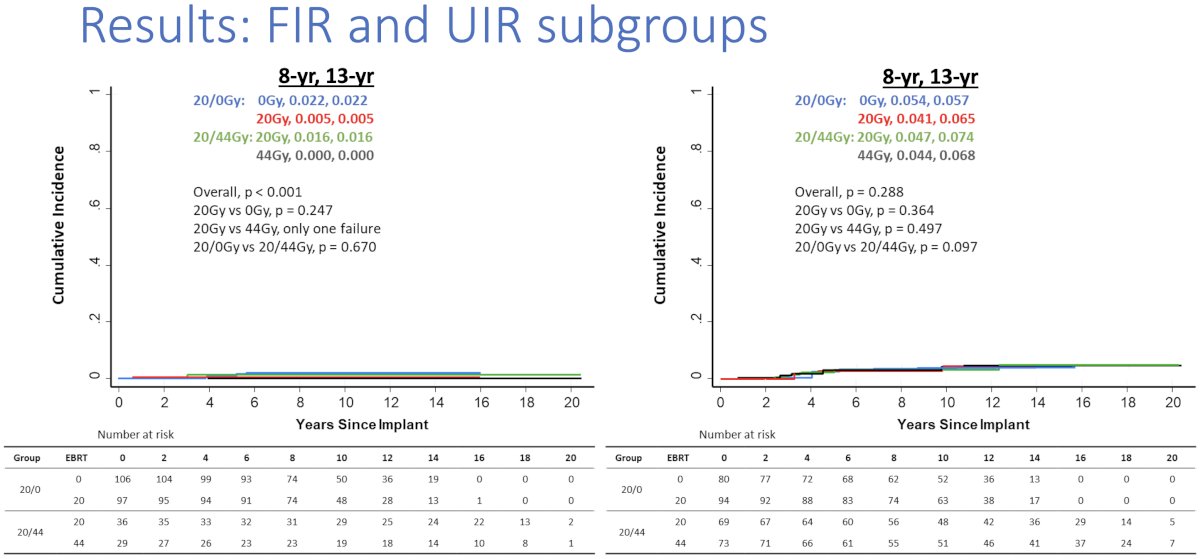
Notably, BED was not associated with biochemical failure in either subgroup.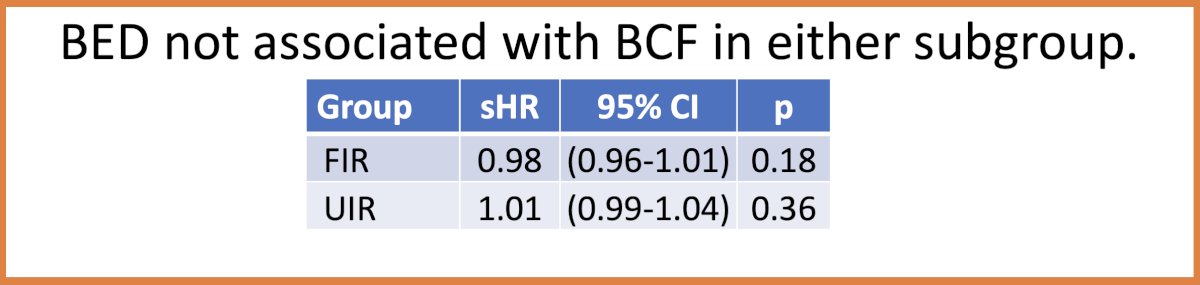
Dr. King concluded his presentation as follows:
- Greater supplemental EBRT dose was not associated with improved outcomes in either trial with long-term follow-up.
- Greater BED did not translate into improved outcomes for the entire cohort, or the unfavorable intermediate-risk subgroup.
- Low rates of biochemical failure for unfavorable intermediate risk were achieved with exclusive Pd-103 implants, high D90, and extra-prostatic seeds.
- Brachytherapy monotherapy should be considered as a standard-of-care option for clinically localized, unfavorable intermediate-risk disease.
Presented by: Martin King, MD, PhD, Director of Brachytherapy Clinical Operations, Senior Physician, Assistant Professor of Radiation Oncology, Harvard Medical School, Dana-Farber Cancer Institute, Boston, MA
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 ASTRO Annual Congress held in Washington, DC between September 29th and October 2nd, 2024
Reference:


