(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between September 29 and October 2 was host to the session EDU 16 - Management of Unfavorable Intermediate-Risk Prostate Cancer: Role of SBRT, Brachytherapy and Androgen Deprivation Therapy. Dr. Cynthia Menard delved into the role of Brachytherapy for Unfavorable Intermediate Risk Prostate Cancer.
The ASCENDE-SBRT, or PR 24 trial, is a randomized controlled trial led by the Canadian Cancer Trials Group (CCTG) focusing on patients with unfavorable intermediate-risk prostate cancer. In this trial, participants are randomized to receive either external beam radiation therapy (EBRT) with a brachytherapy boost (low-dose rate or high-dose rate) or hypofractionated radiation therapy through stereotactic body radiation therapy (SBRT), which involves delivering 25 Gy in 5 fractions to the pelvis and 40 Gy in 5 fractions to the prostate. The trial aims to enroll approximately 710 participants, with progression-free survival as the primary endpoint. Dr. Menard emphasized that the standard of care treatment arm includes EBRT with a brachytherapy boost, while the experimental arm consists of SBRT.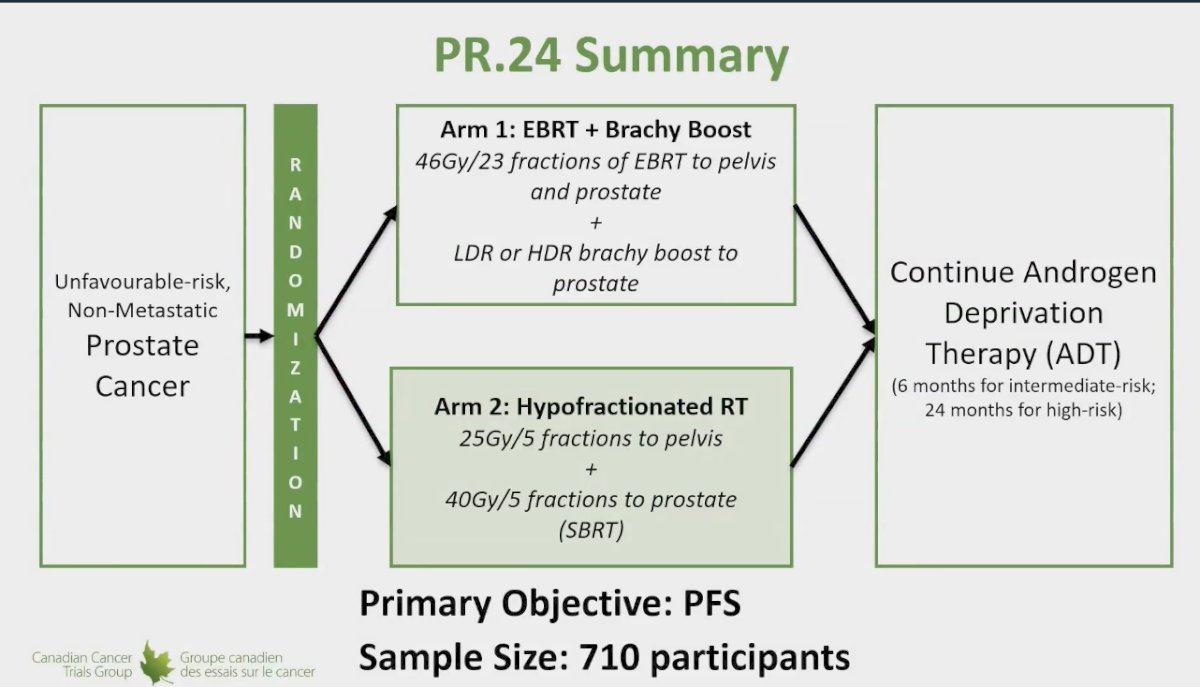
There is compelling evidence of a dose effect in unfavorable intermediate-risk prostate cancer, with nearly all randomized controlled trials evaluating dose escalation demonstrating benefits in biochemical progression-free survival (bPFS). However, most trials have not shown a significant advantage in metastasis-free survival (MFS). This raises the question of why we should pursue dose escalation if MFS benefits are lacking. The justification lies in the improvement of bPFS; since biochemical failure often leads to intensified follow-up, increased patient anxiety, the need for second-line treatments—which may incur additional toxicity—and associated costs, enhancing bPFS remains a critical consideration in treatment planning.
The long-term results of the GETUG-AFU 18 trial were presented at ASCO GU 2024. This phase III randomized trial included patients with high-risk prostate adenocarcinoma (cT3-T4 or PSA ≥ 20 ng/mL or Gleason score ≥ 8-10) who had negative lymph nodes (N0). Participants were randomly assigned in a 1:1 ratio to receive either dose-escalated radiotherapy (80 Gy) or conventional-dose radiotherapy (70 Gy), both accompanied by 3 years of androgen deprivation therapy (ADT). The Kaplan-Meier analysis indicated a significant overall survival advantage for the dose-escalation arm, with a 10-year overall survival (OS) rate of 77.0% in the dose-escalated group compared to 65.9% in the conventional radiotherapy group.1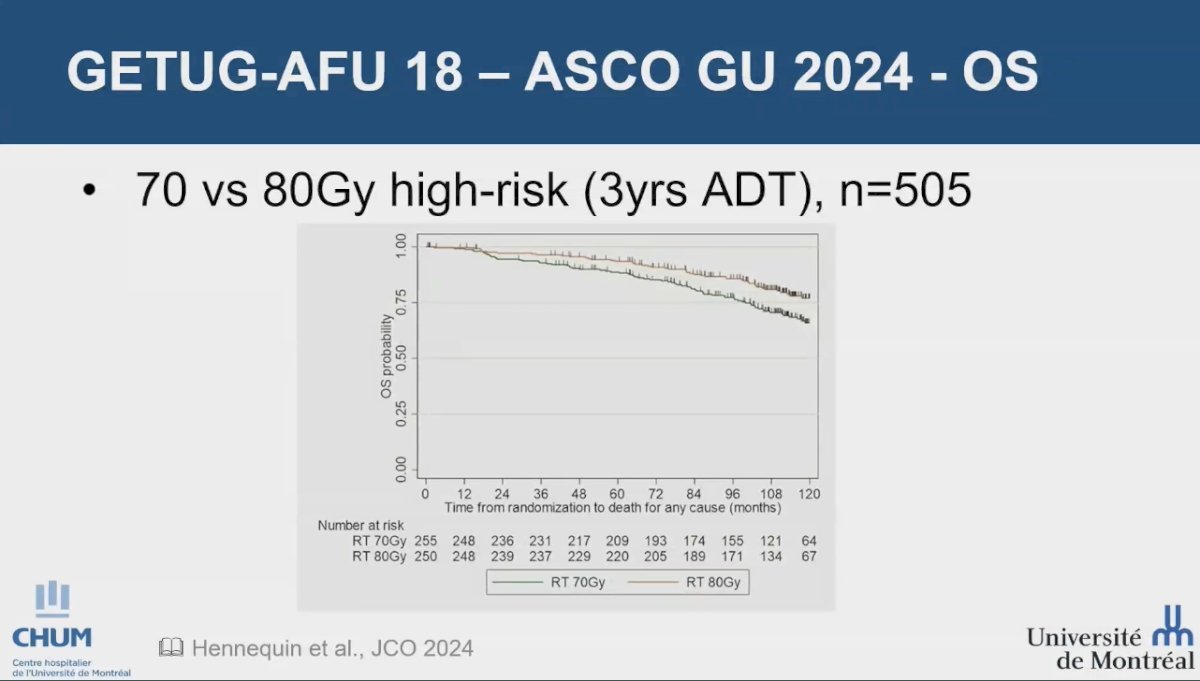
The only study to date demonstrating an improvement in metastasis-free survival (MFS) with dose escalation in prostate cancer is a retrospective cohort study conducted by Kishan and colleagues. This study included 6,004 men with high-risk prostate cancer who were treated with either radical prostatectomy (RP), external beam radiotherapy (EBRT) with androgen deprivation therapy (ADT), or EBRT plus brachytherapy boost (BT) with ADT. The analysis revealed that compared to RP, treatment with EBRT combined with BT (P = 0.03) or EBRT alone (P = 0.01) was associated with significantly improved prostate cancer-specific mortality and MFS (P < 0.001 for both). These findings suggest that dose escalation with brachytherapy is linked to better outcomes in MFS.2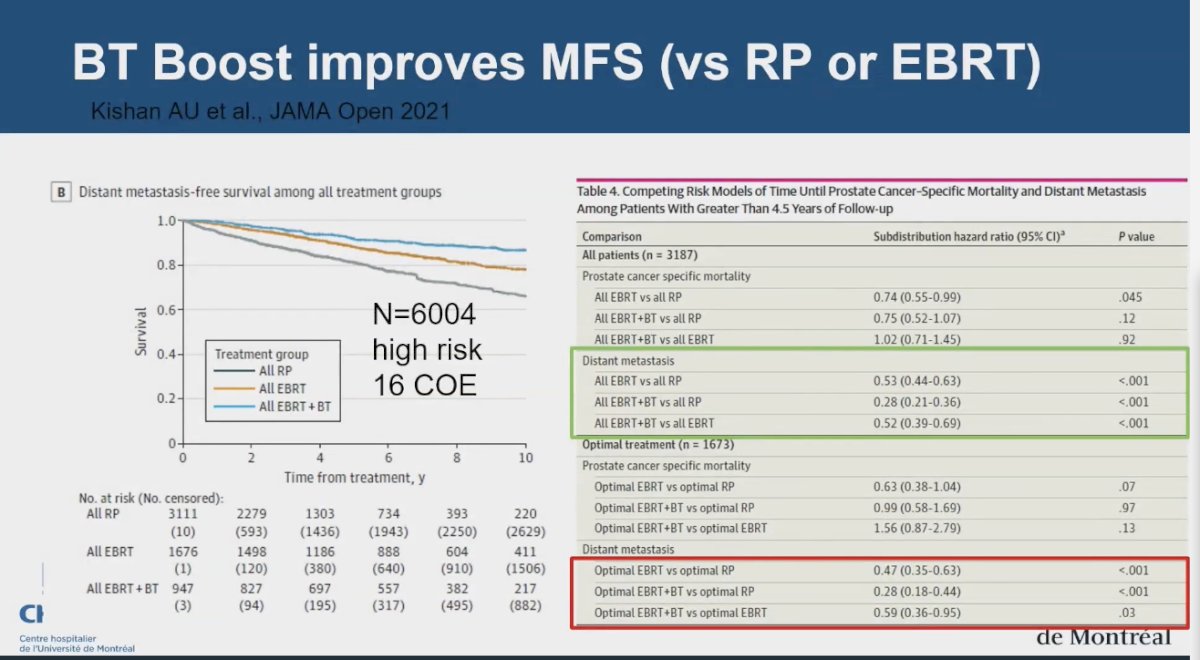
The maximum dose ceiling for dose escalation in prostate cancer treatment has been discussed, with Dr. Menard indicating that the widely accepted threshold is around 80 Gy of EQD2. However, the FLAME study showed a dose effect beyond this limit. In the trial, patients in the standard treatment group received 77 Gy to the entire prostate, while those in the focal boost arm received an additional simultaneous integrated boost of up to 95 Gy to the intraprostatic lesion identified via multiparametric magnetic resonance imaging. This dose escalation was associated with a significant benefit in biochemical disease-free survival (bDFS). It is noteworthy that not all patients in the FLAME trial received an EQD2 dose above 100 Gy; the mean dose was 85 Gy, resulting in an overall mean EQD2 of 95 Gy.3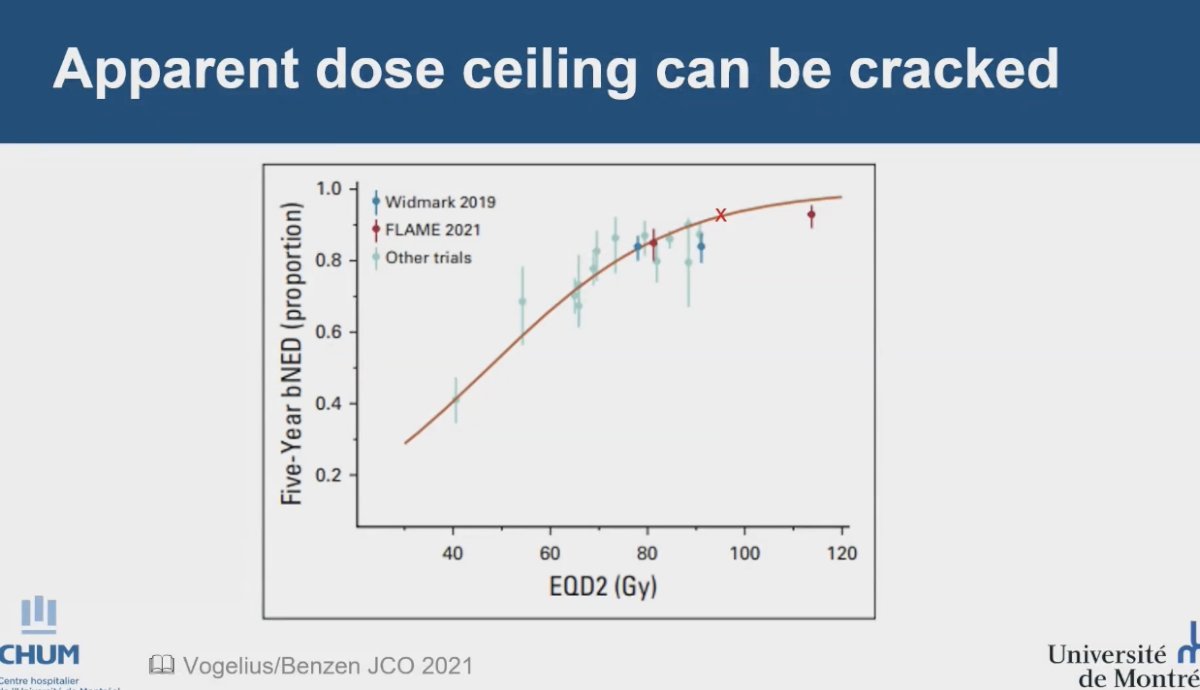
In a substudy of the FLAME trial, patients with high-risk characteristics, particularly those with Gleason Grade (GG) 4 or GG 5, demonstrated a low 5-year disease-free survival (DFS) rate. The results indicated that focal boosting significantly improved DFS in this population, suggesting that achieving a high focal boost dose may be especially advantageous for these patients.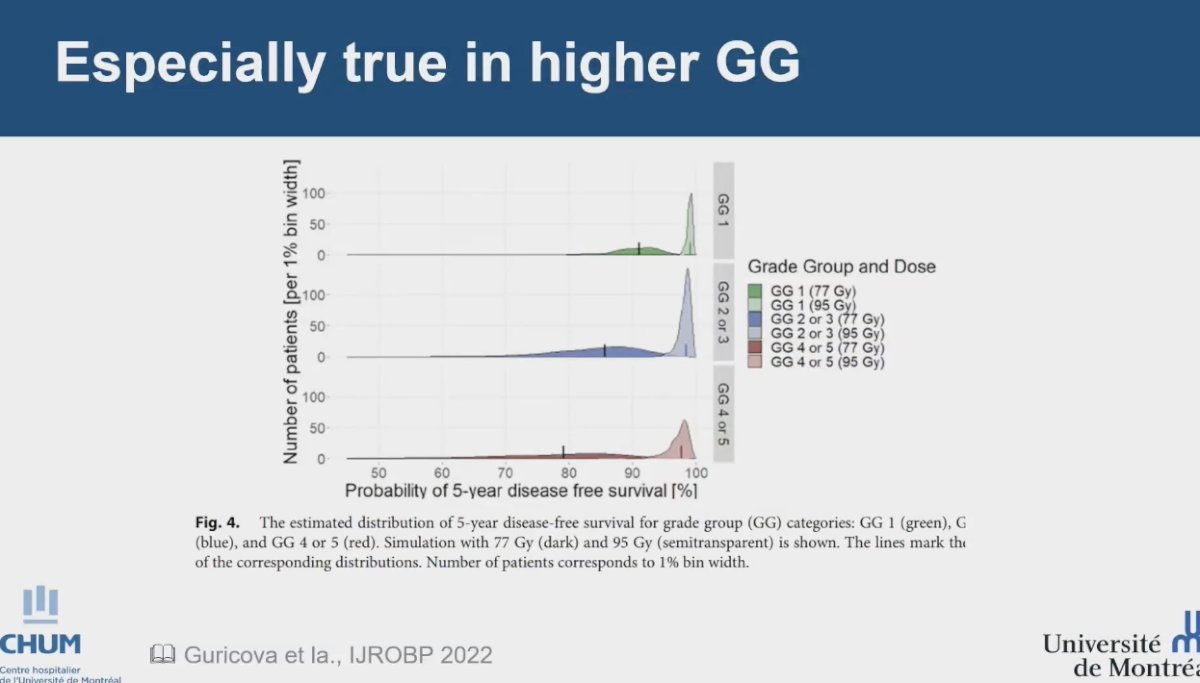
However, dose escalation can lead to increased toxicity in some trials. The ASCENDE-RT trial, a randomized study enrolling 398 men, compared a standard arm that included 12 months of ADT and pelvic irradiation to 46 Gy followed by a dose-escalated external beam radiation therapy (DE-EBRT) boost to 78 Gy, with an experimental arm that utilized a low-dose-rate prostate brachytherapy (LDR-PB) boost. This trial demonstrated a significant benefit of the LDR-PB boost, achieving a 10-year MFS rate of 85% for LDR-PB compared to 67% for DE-EBRT (P < 0.001). However, this benefit came at the cost of increased grade 3 genitourinary (GU) events, with rates of 18.4% for LDR-PB versus 5.2% for DE-EBRT (P < 0.001). As a result, while the extreme dose escalation in this trial showed promising outcomes, it has not been widely adopted as the standard of care globally due to the associated toxicity.4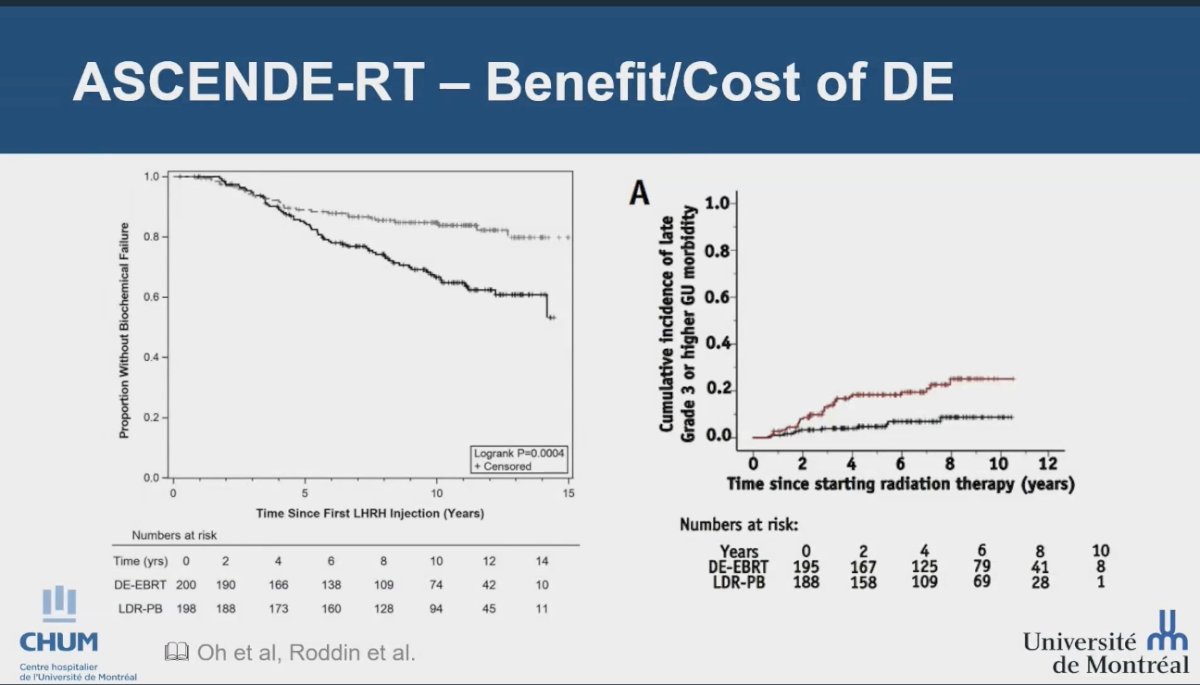
evaluated men with intermediate or high-risk prostate cancer who were treated with combined pelvic external beam radiation therapy (EBRT) and brachytherapy (BT). Participants were randomly assigned to receive either a high-dose-rate (HDR) boost of 15 Gy or a low-dose-rate (LDR) boost of 110 Gy. The trial showed that urinary quality of life (QoL) associated with HDR-BT improved over time, ultimately becoming equivalent to that of LDR after 18 months. However, in the first year, HDR was associated with lower mean International Prostate Symptom Score (IPSS) and urinary QoL scores compared to LDR, indicating an initial benefit in terms of urinary toxicity with HDR treatment.5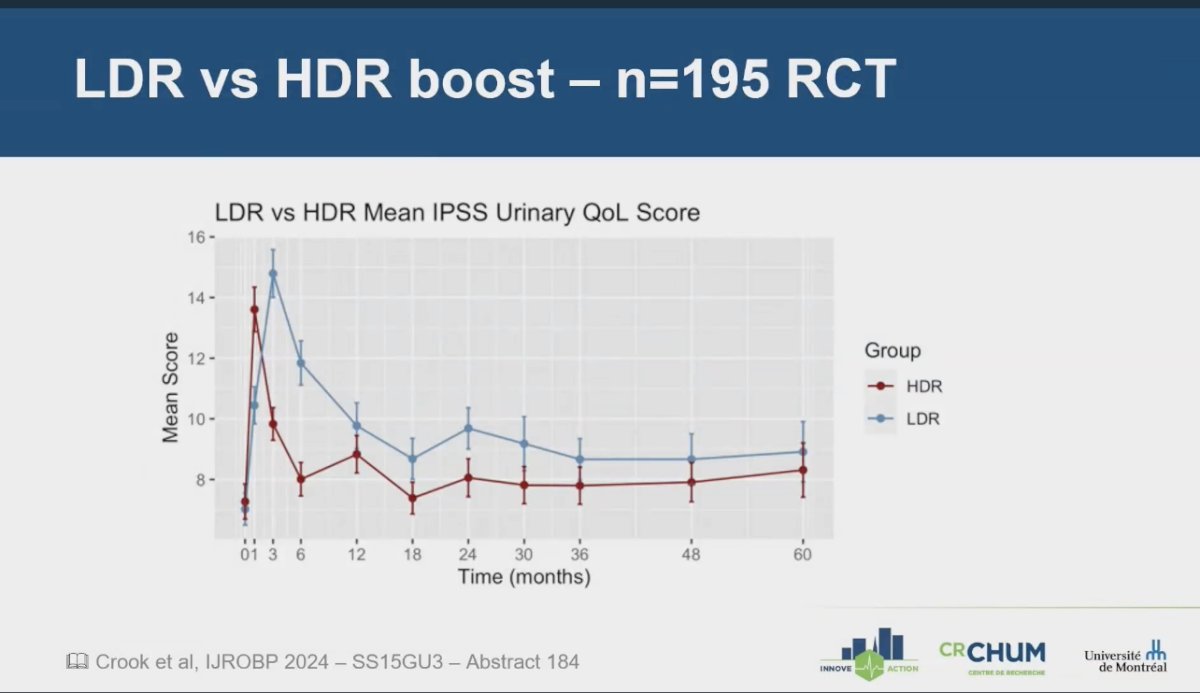
The PCS6 randomized trial compared EBRT combined with an HDR boost against hypofractionated radiotherapy, with all patients receiving neoadjuvant, concurrent, and long-term ADT. In the EBRT + HDR boost group, patients received 46 Gy in 2 Gy fractions to the pelvis, followed by a 15 Gy HDR boost delivered in one fraction within three weeks of EBRT. Conversely, the hypofractionated radiation therapy group received 68 Gy in 2.72 Gy per fraction to the prostate and 45 Gy in 1.8 Gy per fraction to the pelvic lymph nodes. The trial's findings revealed a higher incidence of grade 1 or worse acute gastrointestinal and genitourinary events, as well as a greater frequency of grade 2 or worse acute gastrointestinal events in the hypofractionated radiation therapy group compared to the EBRT + HDR boost group. These results contradicted the initial hypothesis of having more toxicity with the dose-escalation approach, emphasizing the potential benefits of using HDR boost as a safer approach for dose escalation in prostate cancer treatment.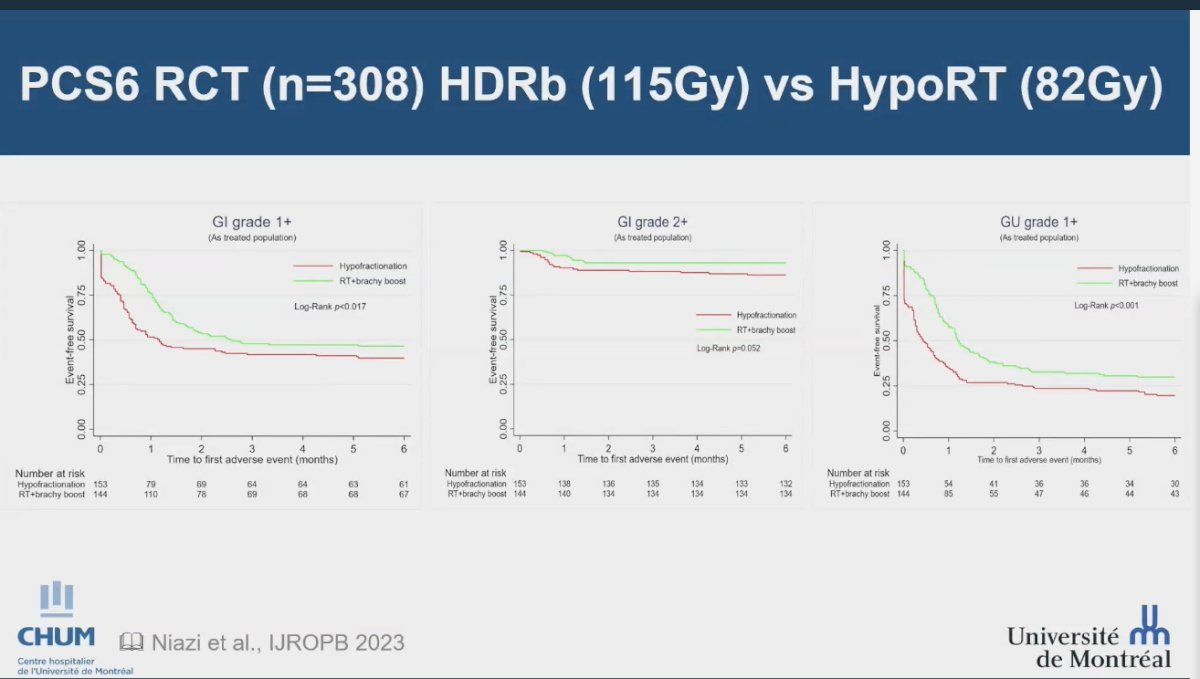
To address the question of the optimal number of Gy per fraction, the ongoing PCS XI phase 3 randomized controlled trial (NCT05820633) is comparing pelvic ultra-hypofractionated radiotherapy (UHF) at 5 Gy per fraction to standard or moderate hypofractionation (1.8–2.15 Gy per fraction). Both treatment regimens include a high-dose-rate (HDR) brachytherapy boost to the prostate and adjacent seminal vesicles, along with androgen deprivation therapy (ADT). The results of this trial will provide valuable insights into the optimal fractionation strategy for prostate cancer treatment.
Dr. Menard revisited the FLAME study, highlighting its role as an isotoxic dosimetry trial. She pointed out the opportunity to model dose-response up to 110 Gy EQD2, demonstrating a therapeutic response up to 100 Gy EQD2. However, it is noteworthy that achieving this dose was not feasible for approximately 50% of the trial participants, particularly when the tumor was located posteriorly. She emphasized the importance of distinguishing between the mean dose to the tumor and the mean dose to the prostate, noting that the dose-response is much sharper and more consistent when considering the mean dose to the tumor, as illustrated in the accompanying graphic. Dr. Menard highlighted that this concept is well established in basic radiation biology, and interestingly, the trial's findings support it with real patient data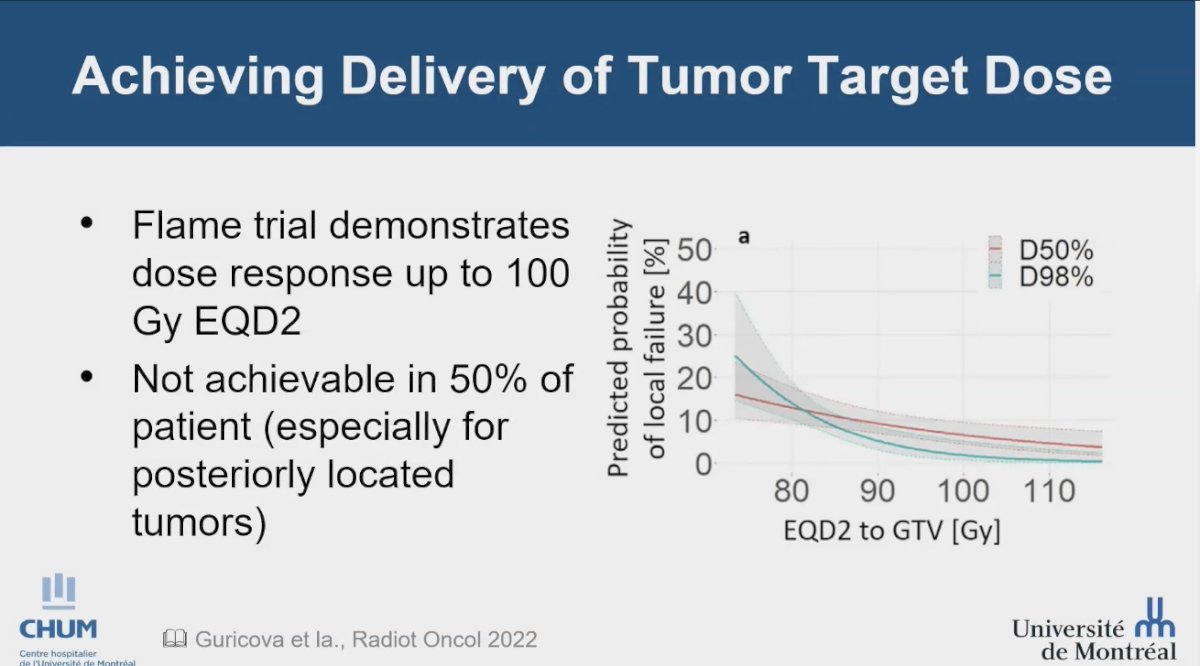 .
.
An alternative strategy for tumor boost could involve using an integrated boost compared to an HDR focal boost. The TARGET trial (NCT01802242) compared an internal boost using Volumetric Modulated Arc Therapy (VMAT) delivering 95 Gy to the planning target volume (PTV, or gross tumor volume [GTV]) in 38 fractions versus an HDR boost of 10 Gy to the PTV (GTV). Notably, Dr. Menard questioned the trial design, which aimed for an EQD2 of 142 Gy. However, the findings revealed equivalent toxicity profiles, with only one Grade 3 event reported in the internal boost group, alongside a remarkable 92% 5-year failure-free survival rate.![The TARGET trial (NCT01802242) compared an internal boost using Volumetric Modulated Arc Therapy (VMAT) delivering 95 Gy to the planning target volume (PTV, or gross tumor volume [GTV]) in 38 fractions versus an HDR boost of 10 Gy to the PTV (GTV)](/images/com-doc-importer/180-astro-2024/astro-2024-role-of-brachytherapy-for-unfavorable-intermediate-risk-prostate-cancer/image-9.jpg)
The question arises: do we really need to irradiate the entire gland? An ongoing Phase 2 trial (NCT03378856) is comparing standard of care, which includes a 15 ± 18 Gy boost plus 25 Gy in 5 fractions of radiation therapy, against a treatment approach involving a 13-15 Gy focal boost with 36.25 ± 40 Gy of stereotactic radiation therapy (sRT). This trial is expected to complete accrual by early 2025, aiming for a total of 110 patients.
Dr. Menard highlighted Abstract 321, which will be presented at the ASTRO meeting by Peter Chung. This study is a large (n=153), single-institution, single-arm cohort that explores a 15 Gy focal HDR boost to 30 Gy in 5 fractions of SBRT. The results of these studies may provide valuable insights into the necessity of irradiating the entire prostate gland.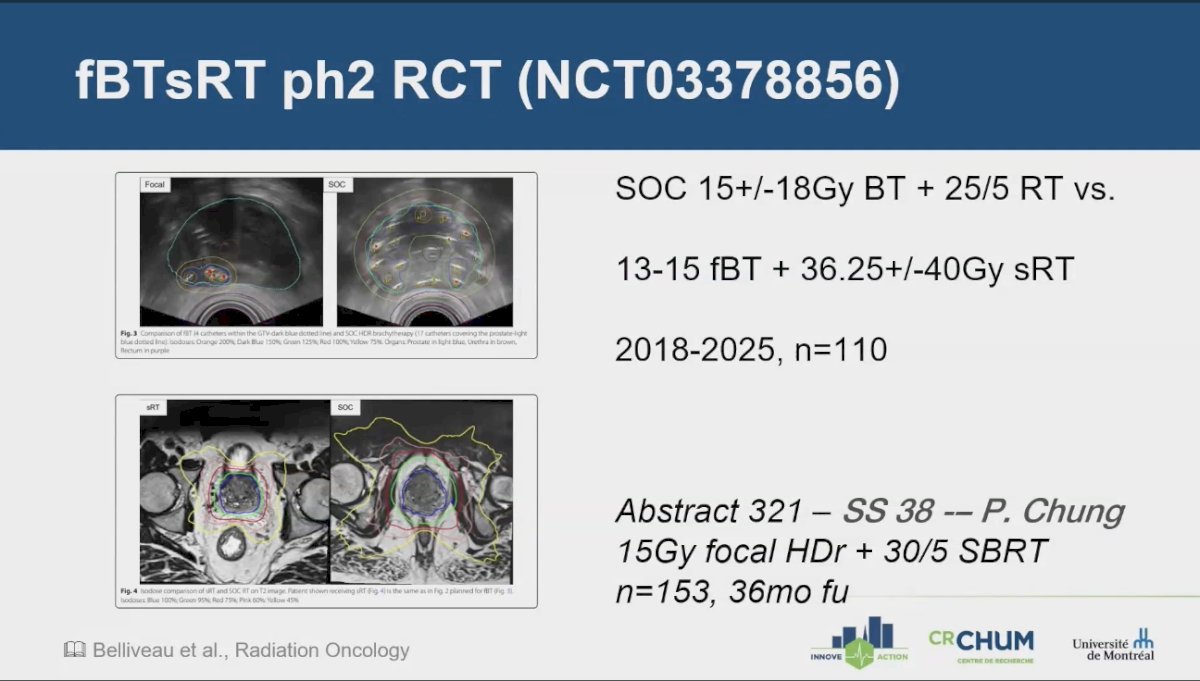
Lastly, Dr. Menard discussed the role of brachytherapy as monotherapy, noting that there is increasing evidence suggesting that an HDR monotherapy approach may be equivalent to EBRT plus an HDR boost. While this data is non-randomized, it presents a compelling alternative strategy, as illustrated in the two studies below: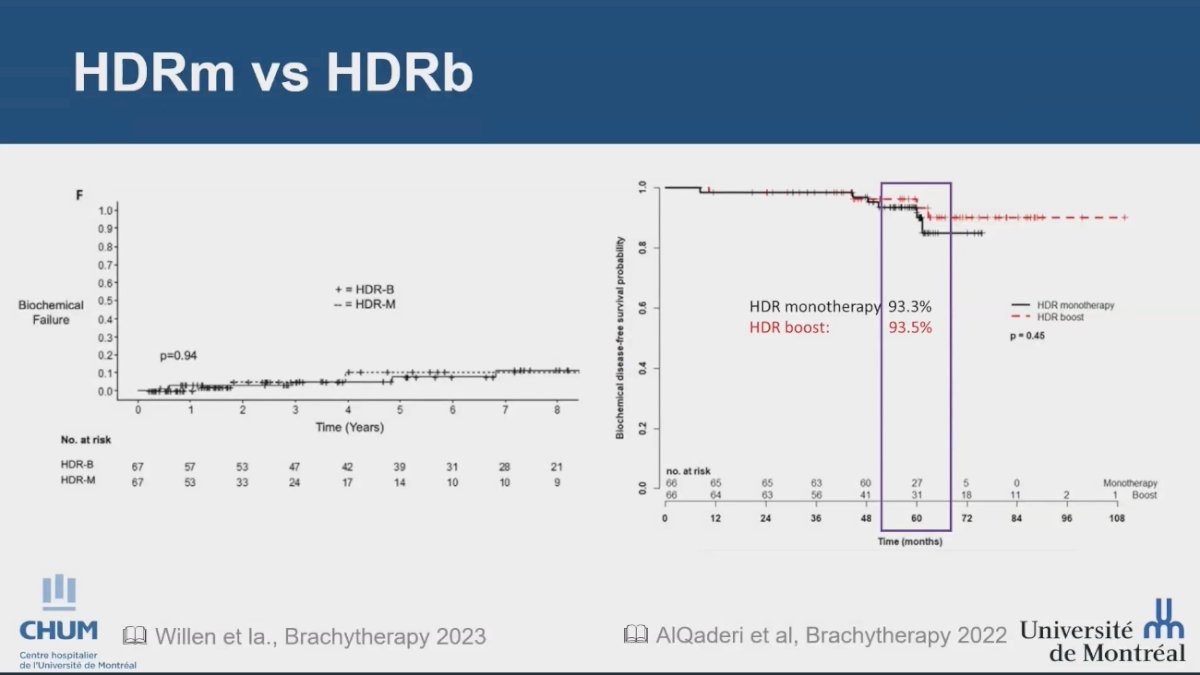
Dr. Menard concludes her presentation with the following take-home messages:
- HDR Brachytherapy technique has been shown to achieve safe dose escalation to the gross tumor volume while maintaining long-term safety for patients. in unfavorable intermediate-risk prostate cancer
- We need to harness the principles of dose-volume effects to enhance precision radiotherapy and optimize clinical outcomes.
Presented by: Cynthia Menard, MD, Chief Department of Radiation Oncology at The University of Montreal Hospital (CHUM), Montreal, Quebec, Canada
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.
References:- Christophe Hennequin et al. Long-term results of dose escalation (80 vs 70 Gy) combined with long-term androgen deprivation in high-risk prostate cancers: GETUG-AFU 18 randomized trial. JCO 42, LBA259-LBA259(2024).
- Kishan AU, Karnes RJ, Romero T, Wong JK, Motterle G, Tosoian JJ, Trock BJ, Klein EA, Stish BJ, Dess RT, Spratt DE, Pilar A, Reddy C, Levin-Epstein R, Wedde TB, Lilleby WA, Fiano R, Merrick GS, Stock RG, Demanes DJ, Moran BJ, Braccioforte M, Huland H, Tran PT, Martin S, Martínez-Monge R, Krauss DJ, Abu-Isa EI, Alam R, Schwen Z, Chang AJ, Pisansky TM, Choo R, Song DY, Greco S, Deville C, McNutt T, DeWeese TL, Ross AE, Ciezki JP, Boutros PC, Nickols NG, Bhat P, Shabsovich D, Juarez JE, Chong N, Kupelian PA, D'Amico AV, Rettig MB, Berlin A, Tward JD, Davis BJ, Reiter RE, Steinberg ML, Elashoff D, Horwitz EM, Tendulkar RD, Tilki D. Comparison of Multimodal Therapies and Outcomes Among Patients With High-Risk Prostate Cancer With Adverse Clinicopathologic Features. JAMA Netw Open. 2021 Jul 1;4(7):e2115312. doi: 10.1001/jamanetworkopen.2021.15312. PMID: 34196715; PMCID: PMC8251338.
- Kerkmeijer LGW, Groen VH, Pos FJ, Haustermans K, Monninkhof EM, Smeenk RJ, Kunze-Busch M, de Boer JCJ, van der Voort van Zijp J, van Vulpen M, Draulans C, van den Bergh L, Isebaert S, van der Heide UA. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021 Mar 1;39(7):787-796. doi: 10.1200/JCO.20.02873. Epub 2021 Jan 20. PMID: 33471548.
- Menne Guricová K, Groen V, Pos F, Monninkhof E, Elias SG, Haustermans K, Smeenk RJ, van der Voort van Zyp J, Draulans C, Isebaert S, van Houdt PJ, Kerkmeijer LGW, van der Heide UA. Risk Modeling for Individualization of the FLAME Focal Boost Approach in External Beam Radiation Therapy for Patients With Localized Prostate Cancer. Int J Radiat Oncol Biol Phys. 2024 Jan 1;118(1):66-73. doi: 10.1016/j.ijrobp.2023.07.044. Epub 2023 Sep 18. PMID: 37725026.
- Rodda S, Tyldesley S, Morris WJ, Keyes M, Halperin R, Pai H, McKenzie M, Duncan G, Morton G, Hamm J, Murray N. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017 Jun 1;98(2):286-295. doi: 10.1016/j.ijrobp.2017.01.008. Epub 2017 Jan 6. PMID: 28433432.
- Crook J, Moideen N, Arbour G, Castro F, Araujo C, Batchelar D, Halperin R, Hilts M, Kim D, Petrik D, Rose J, Cheng JC, Bachand F. A Randomized Trial Comparing Quality of Life After Low-Dose Rate or High-Dose Rate Prostate Brachytherapy Boost With Pelvic External Beam Radiation Therapy. Int J Radiat Oncol Biol Phys. 2024 Sep 1;120(1):59-68. doi: 10.1016/j.ijrobp.2024.02.064. Epub 2024 Mar 16. PMID: 38493901.


