(UroToday.com) The 2024 ASTRO annual meeting included a session on optimizing the therapeutic ratio in prostate cancer, featuring a presentation by Dr. Amar Kishan discussing two-year outcomes from the MIRAGE randomized clinical trial. Dr. Kishan started his presentation by highlighting that planned target volume accounts for possible uncertainties in beam alignment, patient positioning, organ motion, and organ deformation.
In the context of stereotactic body radiotherapy, the major contributor to the planned target volume is prostate motion, and the margin for motion is estimated to be at least 3 mm in the A/P and S/I dimensions with Volumetric modulated arc therapy (VMAT). However, setup uncertainties can persist due to the intrinsic nature of treatment, but also due to residual error introduced by an MRI-CT fusion in contouring and errors introduced during on-table registration. Thus, a reasonable minimum margin for prostate stereotactic body radiotherapy with VMAT would be ~4 mm.
Previously, the MIRAGE trial showed that aggressive margin reduction using MRI guidance has been shown to reduce acute grade 2+ genitourinary and gastrointestinal toxicity following prostate stereotactic body radiotherapy.1 Additional benefits of MRI-guided prostate radiotherapy include potentially enhanced precision of urethra contouring and no need for fiducials or X-ray dose from IGRT. At the 2024 ASTRO annual meeting, Dr. Kishan and colleagues reported the 2-year patient-reported quality of life outcomes and physician-scored toxicity from a phase III randomized trial comparing MRI-guided stereotactic body radiotherapy versus CT-guided stereotactic body radiotherapy.
In this trial, planning margins of 4 mm (CT-arm) and 2 mm (MRI-arm) were placed around the prostate and proximal seminal vesicles, and this volume received 40 Gy in five fractions. Acute toxicity endpoints that were previously reported favoring MRI guidance included [1]:
- Acute grade 2+ genitourinary toxicity reduction: 43.4% versus 24.4%
- Acute grade 2+ gastrointestinal toxicity reduction: 10.5% versus 0%
- Lower proportion of large (ie. >= 15 point) IPSS increases: 19.8% versus 6.4%
- Lowe proportion of significant (ie. >= 12 point) EPIC-26 bowel domain decrement: 50% versus 25%
The pre-specified secondary endpoints included patient-reported Expanded Prostate Cancer Index Composite-26 (EPIC-26) scores and Sexual Health Inventory for Men (SHIM) scores, as well as the cumulative incidence of late grade 2+ genitourinary and gastrointestinal toxicity. The proportion of patients experiencing a decrement in any given quality of life domain exceeding 2x the minimal clinically important difference threshold for the domain at any time point from 6-24 months was compared using the Chi-square test. The 2x minimal clinically important difference thresholds were 14 points for urinary irritative, 12 points for bowel, and 10 points for SHIM. Times to first grade 2 genitourinary or gastrointestinal toxicity were estimated using the Kaplan-Meier method and the log-rank test was used to compare the treatment groups.
Between May 2020 and October 2021, 156 patients (n = 77 CT and n = 79 MRI) were randomized. Overall, 81% of patients had NCCN unfavorable intermediate-, high, or very high-risk disease, 44% had placement of a spacer, and 27% had nodal radiation:
Cumulative rates of both grade 2+ genitourinary and gastrointestinal toxicity were lower in the MRI-guidance arm (genitourinary, 27% [95% CI 41-63%] versus 51% [95% CI 41-63%], p = 0.004 and gastrointestinal, 1.4% [95% CI 0.2-9.6%] versus 9% [95% CI 4.6-19%], p = 0.025 by log-rank comparing incidence functions):
Patients treated with MRI guidance had significantly lower rates of 2x minimal clinically important difference in the urinary irritative (19.2% vs. 35.3%, p = 0.03) and bowel (26% vs. 4%, p = 0.04) domains:
Among patients not receiving ADT, patients treated with MRI guidance had significantly lower rates of 2x minimal clinically important difference in SHIM scores (22% vs. 53%, p = 0.04):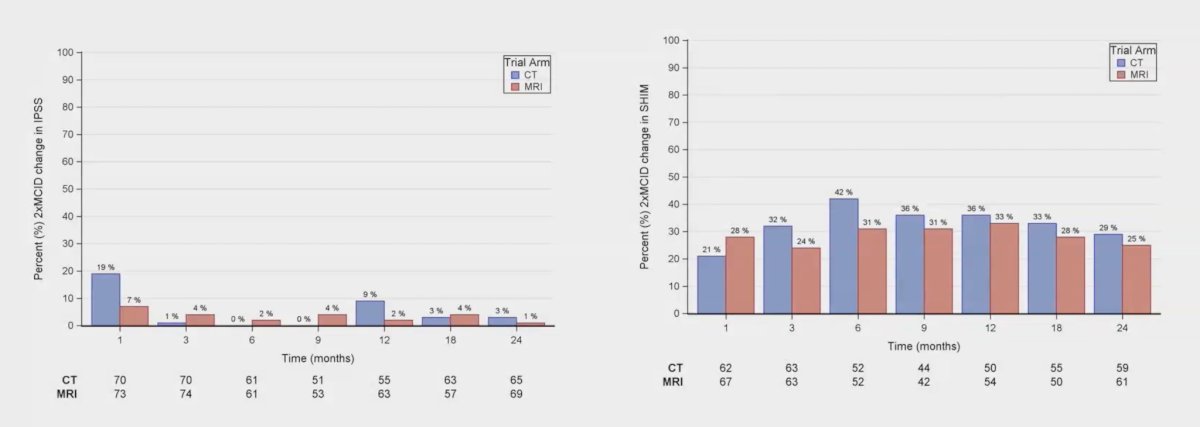
Finally, assessing logistic regression for the patient-reported outcomes, both bowel (OR 0.44, 95% CI 0.21 – 0.94, p = 0.03) and SHIM (OR 0.37, 95% CI 0.15 – 0.91, p = 0.03) were significantly improved with MRI vs CT guided radiotherapy: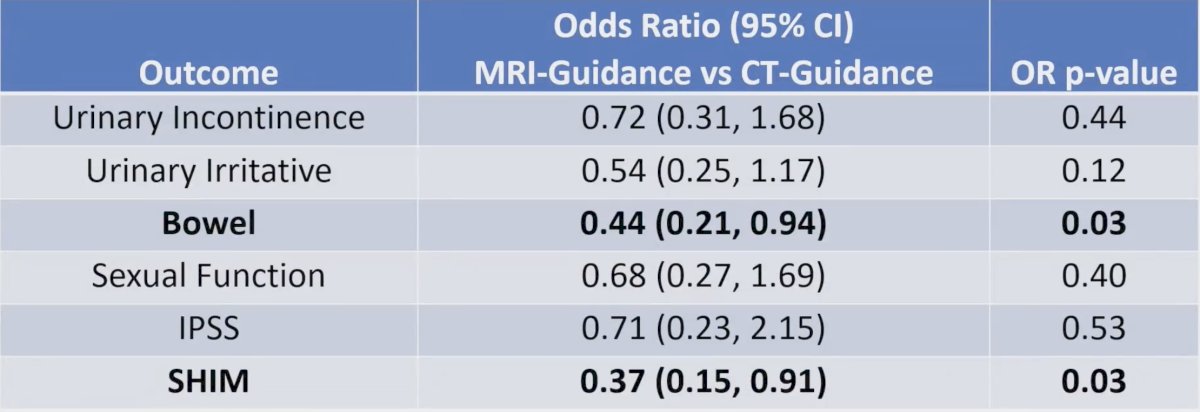
Dr. Kishan concluded his presentation discussing two-year outcomes from the MIRAGE randomized clinical trial with the following take-home points:
- Patients receiving MRI-guided stereotactic body radiotherapy had significantly lower cumulative incidences of grade 2+ genitourinary and gastrointestinal toxicity through two years
- On logistic regression, the odds of experiencing clinically relevant decrements in bowel quality of life or SHIM scores were significantly lower in patients treated with MRI-guided stereotactic body radiotherapy.
- The toxicity benefits of aggressive margin reduction persist beyond the acute phase post-SBRT
Presented by: Amar Kishan, MD, Radiation Oncologist, UCLA Radiation Oncology, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.
Reference:


