(UroToday.com) The 2024 ASTRO annual meeting included a session on optimizing the therapeutic ratio in prostate cancer, featuring a presentation by Dr. Vishal Dhere discussing volumetric changes and acute toxicity with 68Ga PSMA PET/CT versus 18F-Fluciclovine PET/CT guided post-prostatectomy radiation from the EMPIRE-2 trial.
68Ga- PSMA and 18F-Fluciclovine PET/CT are approved in post-prostatectomy patients with biochemical recurrence. Previously, EMPIRE-1 showed that 18F-Fluciclovine PET/CT guided post-prostatectomy radiation improved 3-year failure-free survival (75.5% versus 63.0%, p < 0.01):1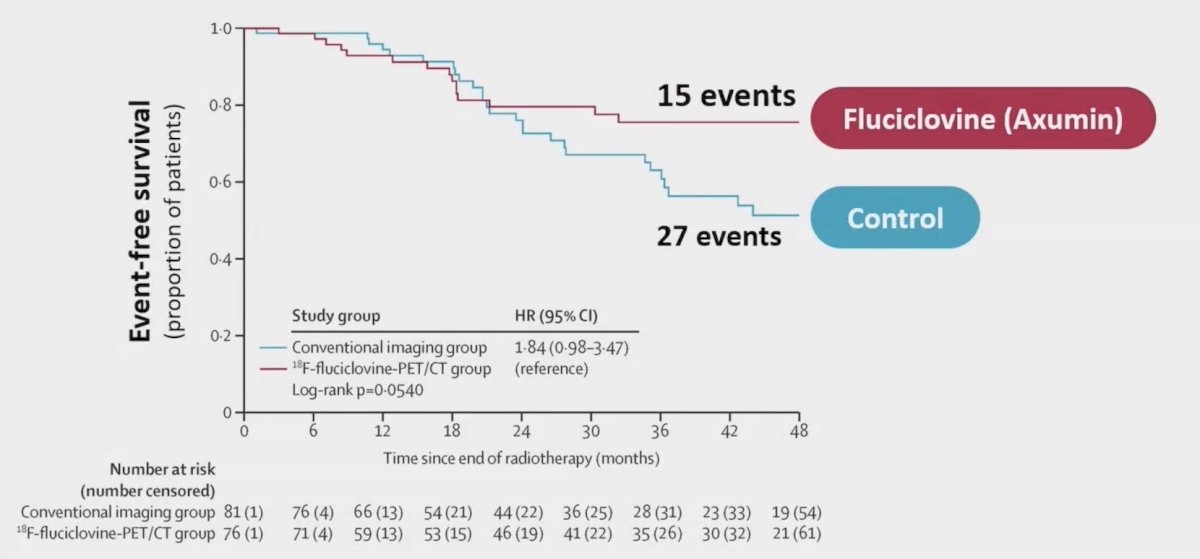
EMPIRE-1 also showed modest changes in prostate bed and pelvic target volumes: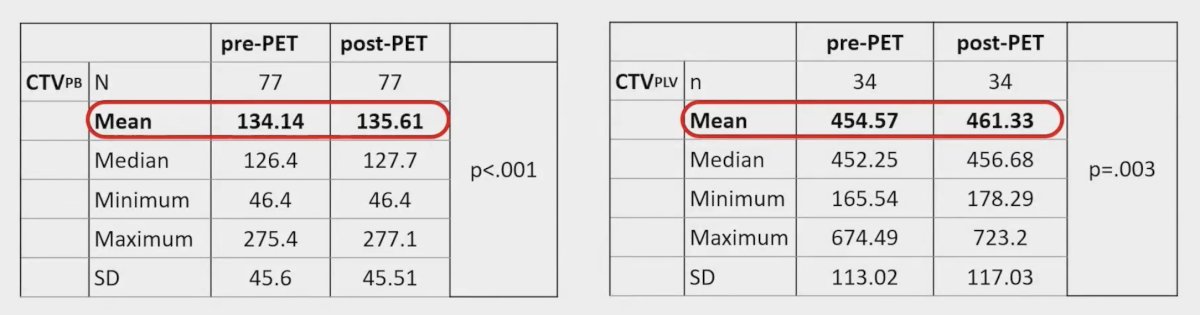
Dr. Dhere and colleagues evaluated changes in radiation therapy target volume or acute toxicity using 68Ga PSMA PET/CT versus 18F-Fluciclovine PET/CT in a randomized fashion. Moreover, they hypothesized that both 18F-Fluciclovine PET/CT and 68Ga PSMA PET/CT guided radiation therapy would (i) significantly change pre-PET radiation therapy volumes and (ii) show similar toxicity.
EMPIRE-2 was a prospective, randomized trial comparing 18F-Fluciclovine PET/CT (Arm 1) and 68Ga PSMA PET/CT (Arm 2)-guided post-prostatectomy radiation therapy in patients with detectable PSA after prostatectomy. They assessed radiation therapy volumes before (pre-) and after (post-) PET incorporation using the Wilcoxon signed-rank tests. Treatment volumes were rigidly defined based on PET uptake and simultaneous integrated boost to areas of uptake in the prostate bed (70-76 Gy) or pelvis (54-56 Gy) was allowed: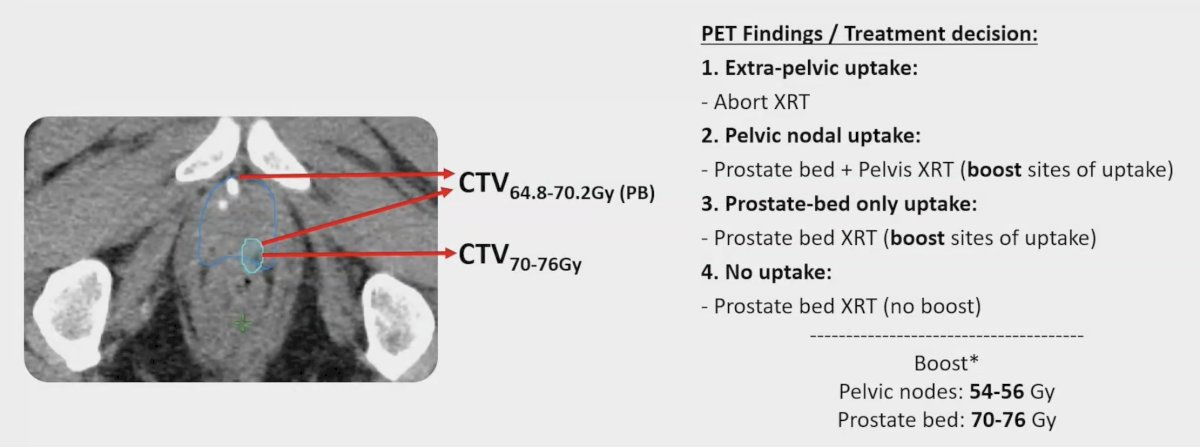
Volumes assessed included:
- Prostate bed (CTV prostate bed)
- Pelvic lymph nodes (CTV pelvic lymph nodes)
- Volume at 65Gy (V65Gy) for bladder-CTV & rectum
Acute CTCAE v5.0 toxicity was assessed <30 days from treatment completion by Fisher’s Exact tests. There were 140 patients enrolled, with 70 randomized to each arm: 11 Arm 1 and 10 Arm 2 patients did not receive radiation on study (due to extrapelvic uptake or consent withdrawal) and were excluded: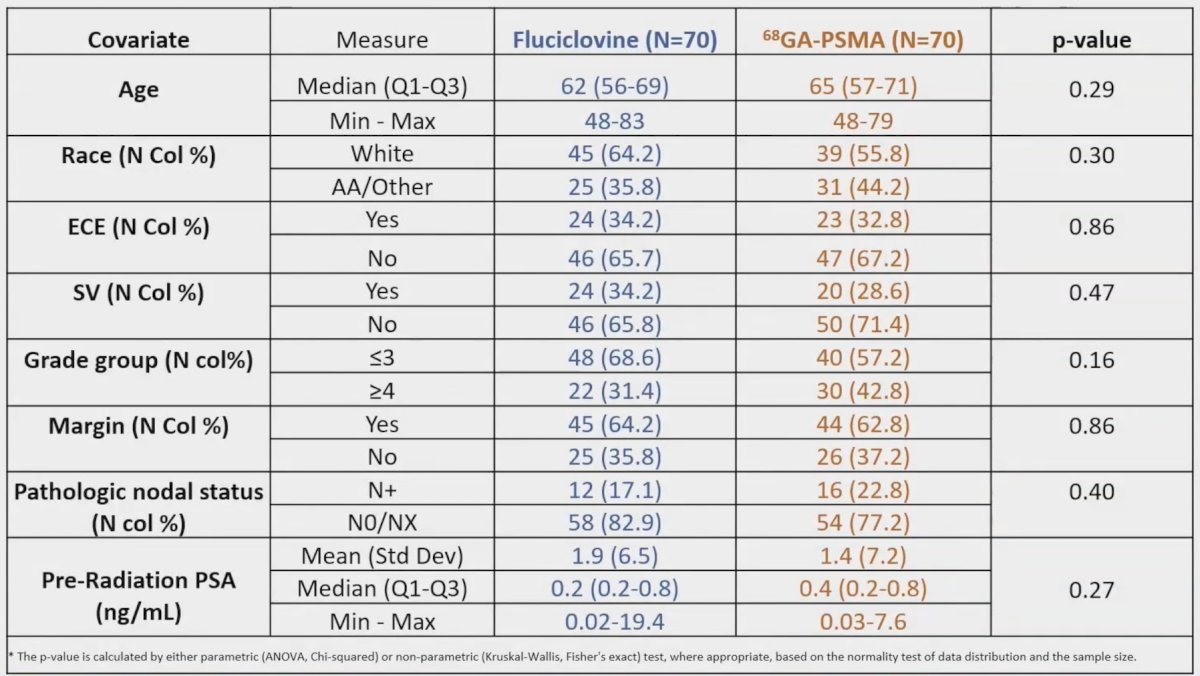
There was no significant difference in initial target volumes between arms (p > 0.05 for all CTV measures):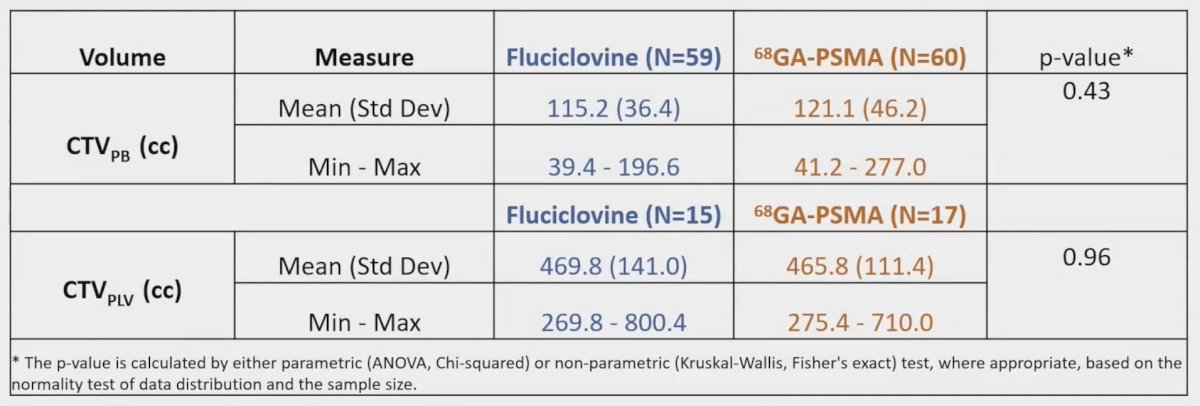
18F-Fluciclovine PET/CT significantly increased mean CTV prostate bed (115.20 cc versus 115.74 cc, pre- versus post-; p < 0.01) and CTV pelvic lymph nodes (469.73 cc versus 472.73 cc, pre- versus post-; p < 0.01) volumes. 68Ga PSMA PET/CT also significantly increased mean CTV prostate bed (121.20 cc versus 121.40 cc, pre- versus post-; p < 0.01) and CTV pelvic lymph nodes (465.80 cc versus 466.80 cc, pre- versus post-; p < 0.01) volumes. All patients with PET uptake in the prostate bed and/or pelvis received simultaneous integrated boosts. More 18F-Fluciclovine PET/CT patients received prostate bed boosts (47/59 patients versus 27/60 patients, p < 0.01) but there was no significant difference in proportion receiving pelvic nodal boosts (10/15 patients versus 9/17 patients, 18F-Fluciclovine PET/CT versus 68Ga PSMA PET/CT; p = 0.97). All patients met rectal V65Gy < 35%; 5 patients in the 68Ga PSMA PET/CT arm and 3 patients in the 18F-Fluciclovine PET/CT arm exceeded bladder-CTV V65Gy < 40%. Rates of grade 2 genitourinary (17.0% versus 6.7%, 18F-Fluciclovine PET/CT versus 68Ga PSMA PET/CT; p = 0.15) and gastrointestinal (5.1% versus 1.7%, 18F-Fluciclovine PET/CT versus 68Ga PSMA PET/CT; p = 0.53) toxicity were not significantly different between arms, with no grade 3+ events: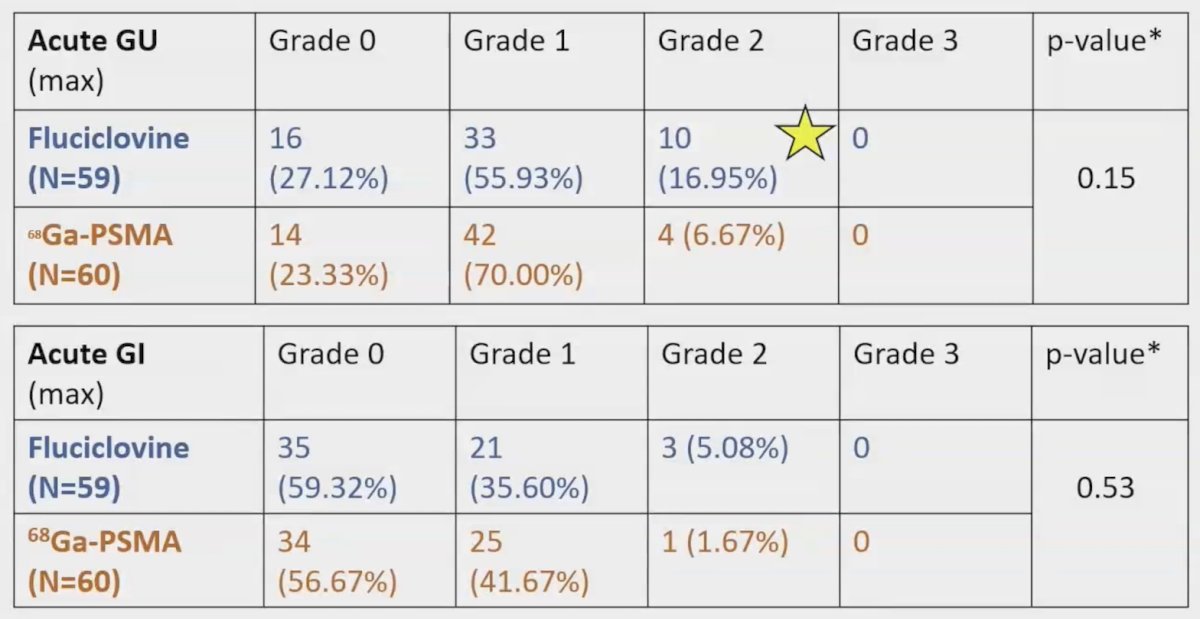
Dr. Dhere concluded his presentation discussing volumetric changes and acute toxicity with 68Ga PSMA versus 18F-Fluciclovine PET/CT guided post-prostatectomy radiation from EMPIRE-2 with the following take-home points:
- Though both 68Ga PSMA PET/CT and 18F-Fluciclovine PET/CT integration increased target volumes, significantly more 18F-Fluciclovine PET/CT patients received prostate bed boosts
- Planning directives were met for most patients and acute toxicity was mild in both arms; toxicity was no great than EMPIRE-1
- Analysis of the primary endpoint (2-year failure free survival) will soon be available
Presented by: Vishal Dhere, MD, Radiation Oncologist, Emory University, Atlanta, GA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.
Reference:


