(UroToday.com) The 2024 ASTRO annual meeting included a session on optimizing the therapeutic ratio in prostate cancer, featuring a presentation by Dr. Carlton Johnny discussing focal MR-guided HDR brachytherapy boost combined with stereotactic body radiotherapy for localized prostate cancer. The objective of this study was to describe a population of patients who may benefit from focal dose escalation with early outcome measures.
In this study, eligible patients included intermediate-risk and high-risk prostate cancer patients with visible gross disease (at 1 or more intraprostatic sites) comprising less than 33% of the total prostate volume on diagnostic MRI. Patients underwent either a 15 Gy single fraction MR-guided HDR focal boost to the gross disease followed by stereotactic body radiotherapy, 30 Gy/5 fractions for intermediate-risk disease (prostate only), or 30 Gy/5 fractions for high-risk disease (prostate + pelvic nodes). ADT was administered based on risk category. The protocol overview for this trial is as follows: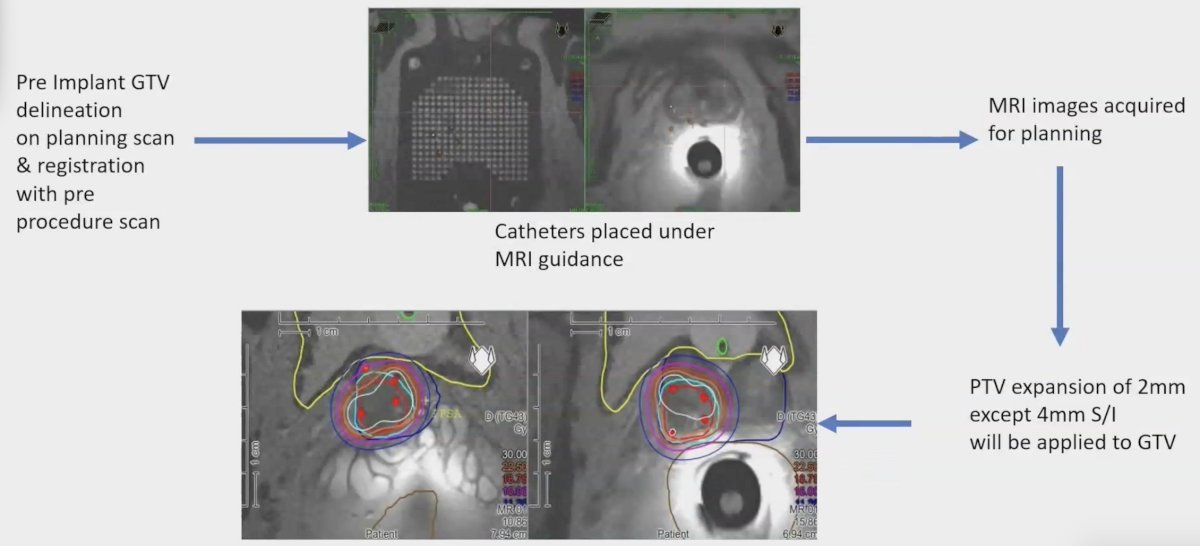
Outcomes included PSA, toxicity (CTCAE v4), and Health-Related Quality of Life (HRQoL) assessments (Expanded Prostate Cancer Index Composite) and were conducted at baseline, then at 1, 3, and 6 months, followed by 1, 2, 3, and 5 years. At a minimum of two years post-radiation, patients underwent a prostate MRI with or without a systematic biopsy to assess local disease status: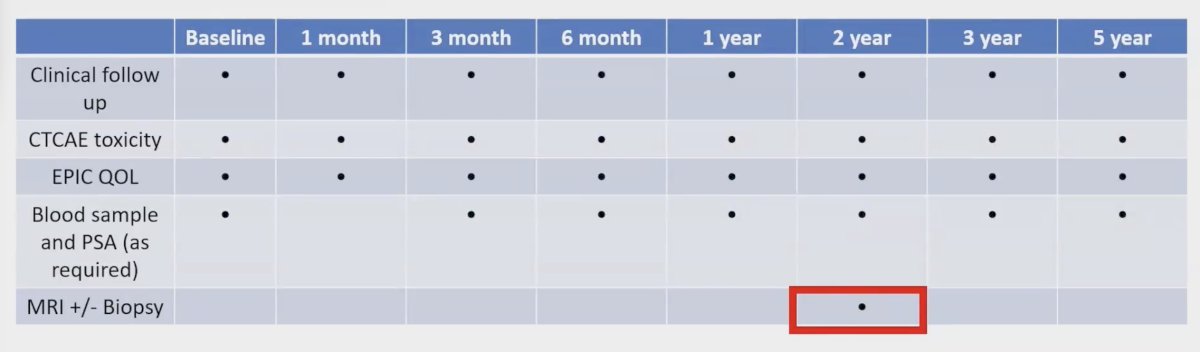
Biochemical failure was defined according to the Phoenix definition (2+ nadir PSA) and restaging was only if the patient was symptomatic or at the time of biochemical failure.
Between 2017 and 2023, there were 153 patients enrolled, with 128 (84%) having intermediate risk and 25 (16%) having high-risk disease. The median age was 69 years old (range: 49-81), and the median PSA was 8 ng/ml (range: 2-53). ADT was administered in 46 (30%) patients. Among the intermediate-risk patients who received ADT (25 patients), 21 (84%) patients received >= 6 months of ADT, while 4 (16%) patients received > 6 months of ADT. Of the 21 patients from the high-risk group, 3 (14%) patients received 6 months of ADT, and 18 (86%) received > 6 months of ADT. Hydrogel spacer insertion was used in 72 (47%) patients: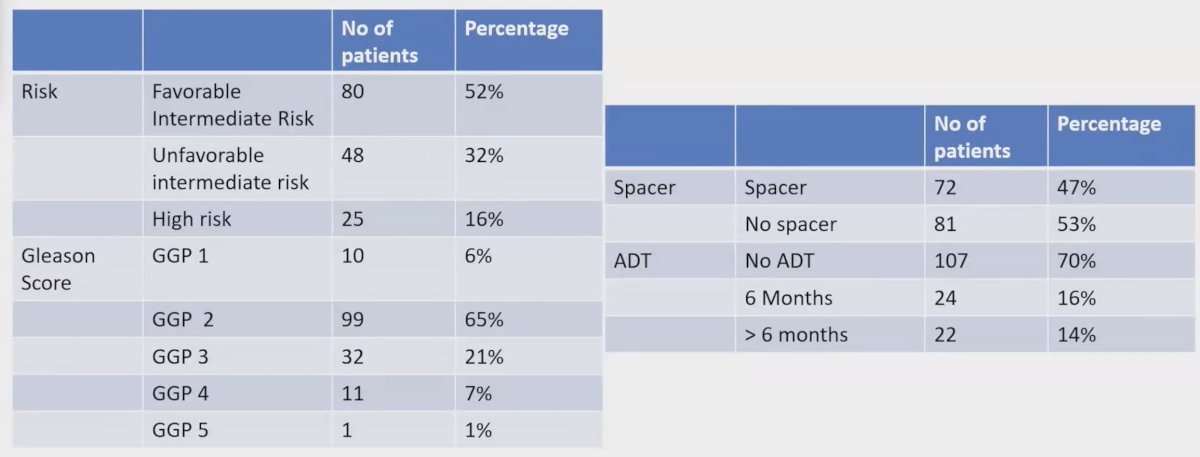
Median follow-up was 36 months (range: 2-79) and the median PSA nadir in patients without ADT was 0.35 ng/mL (range: 0.01-4.75). Overall, the 3-year biochemical control rate was 96.5%, with 96% and 100% in the intermediate-risk and high-risk groups, respectively: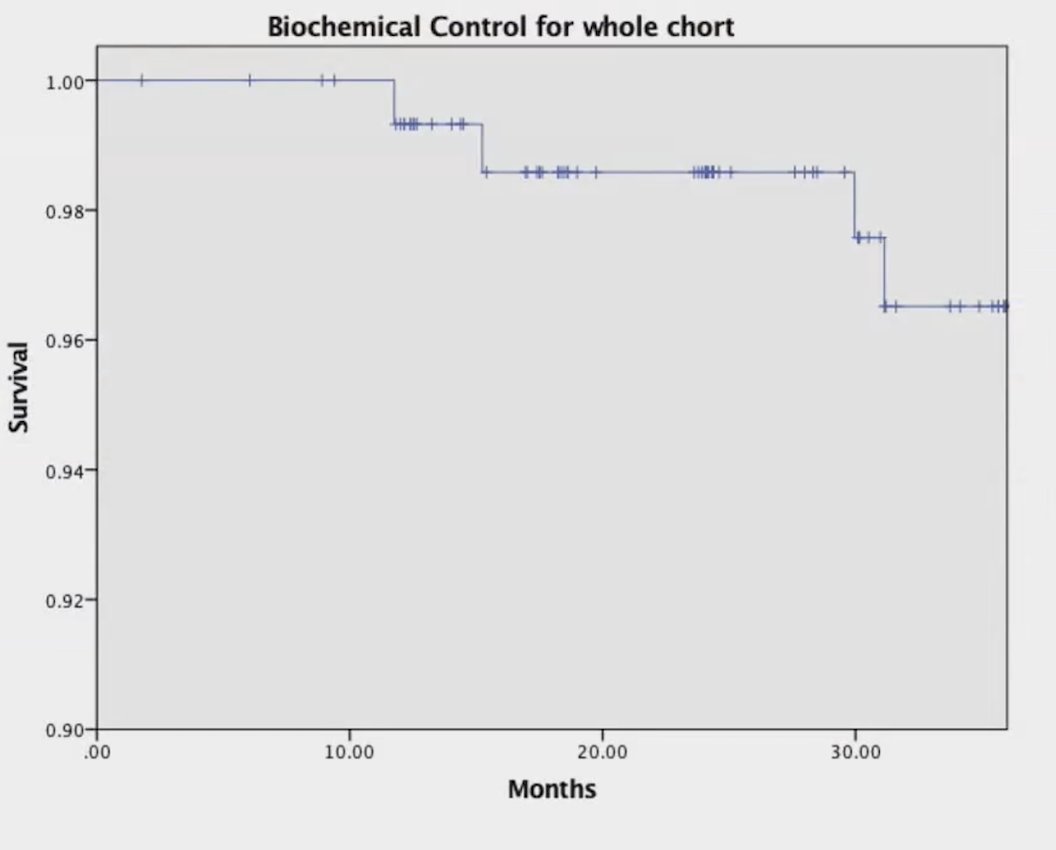
No isolated local failure was observed at 3 years. 3 patients experienced local failure in conjunction with nodal relapse, and one patient had associated regional and distant failure. No isolated regional or distant failures occurred. One month after treatment, the incidence of acute grade > 2 genitourinary and gastrointestinal toxicities were 13% and 2.6%, respectively. By 6 months, these rates decreased to 2.6% and 1.4% for genitourinary and gastrointestinal toxicity, respectively. Late grade 2+ genitourinary and gastrointestinal toxicities at 1 year remained consistent at 2.6% and 0.7%:
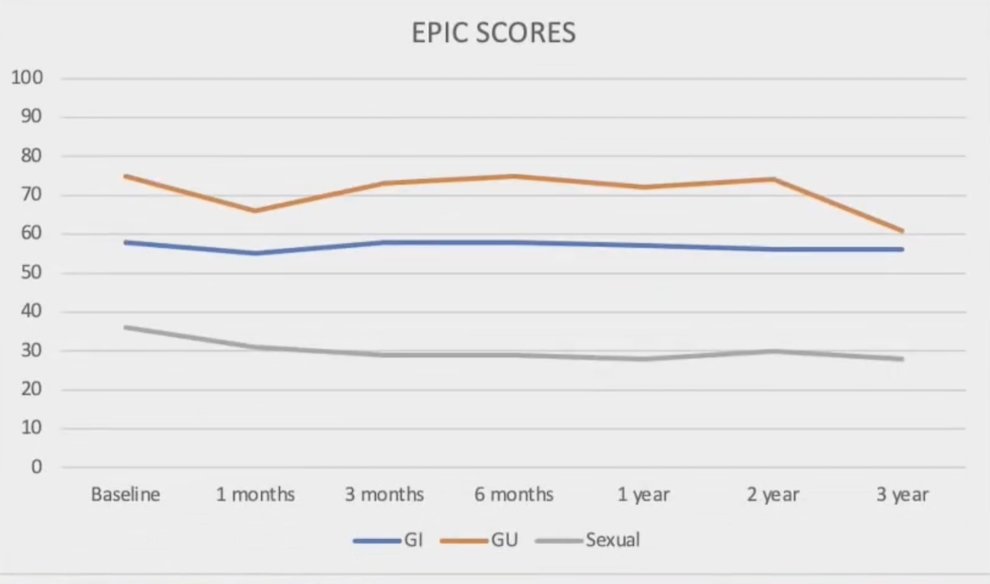
Notably, only one patient encountered severe toxicity (grade 3 genitourinary - hematuria attributed to radiation cystitis at 6 months), which subsequently resolved. Patients with a rectal spacer experienced slightly lower gastrointestinal toxicity, and not statistically significant. No difference in gastrointestinal toxicity was seen in patients who received pelvic nodal irradiation when compared to prostate-only patients.
Dr. Johnny concluded his presentation by discussing focal MR-guided HDR brachytherapy boost combined with stereotactic body radiotherapy for localized prostate cancer with the following take-home points:
- Focal MR-guided HDR brachytherapy combined with stereotactic body radiotherapy demonstrated favorable tolerability and promising local and biochemical control
- Treatment was safe with acceptable patient-reported outcomes
Dr. Johnny noted several important challenges and considerations:
- These results reflect early outcomes based on a short follow-up period
- Current outcomes are predominantly influenced by the intermediate-risk cohort
- As follow-up duration extends, the likelihood of identifying additional treatment failures may increase
- Long-term oncological and toxicity data are awaited
Presented by: Carlton Johnny, MD, Princess Margaret Cancer Center, University Health Network, Toronto, Ontario, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.


