(UroToday.com) The 2024 ASTRO annual meeting included a session on optimizing the therapeutic ratio in prostate cancer, featuring a presentation by Dr. Andre Gouveia discussing early results of PBS, a phase II trial assessing stereotactic body radiotherapy versus conventional fractionation external beam radiotherapy boost for unfavorable intermediate and high-risk prostate cancer.
Treatment of high-risk prostate cancer usually involves pelvic conventional fractionation-external beam radiotherapy, followed by conventional fractionation-external beam radiotherapy boost to prostate in combination with ADT. PBS (NCT03380806) is a randomized phase II trial that evaluated stereotactic body radiotherapy boost after pelvic conventional fractionation-external beam radiotherapy in patients with unfavorable intermediate and high-risk prostate cancer.
Patients with localized unfavorable intermediate and high-risk prostate cancer, from two Ontario radiotherapy facilities, were randomized (1:1) to conventional fractionation-external beam radiotherapy boost (32-34 Gy in 15-17 daily fractions) or stereotactic body radiotherapy boost (19.5-21 Gy delivered in 3 weekly fractions) following pelvic conventional fractionation-external beam radiotherapy (45-46 Gy over 23-25 daily fractions). The trial design of PBS is as follows: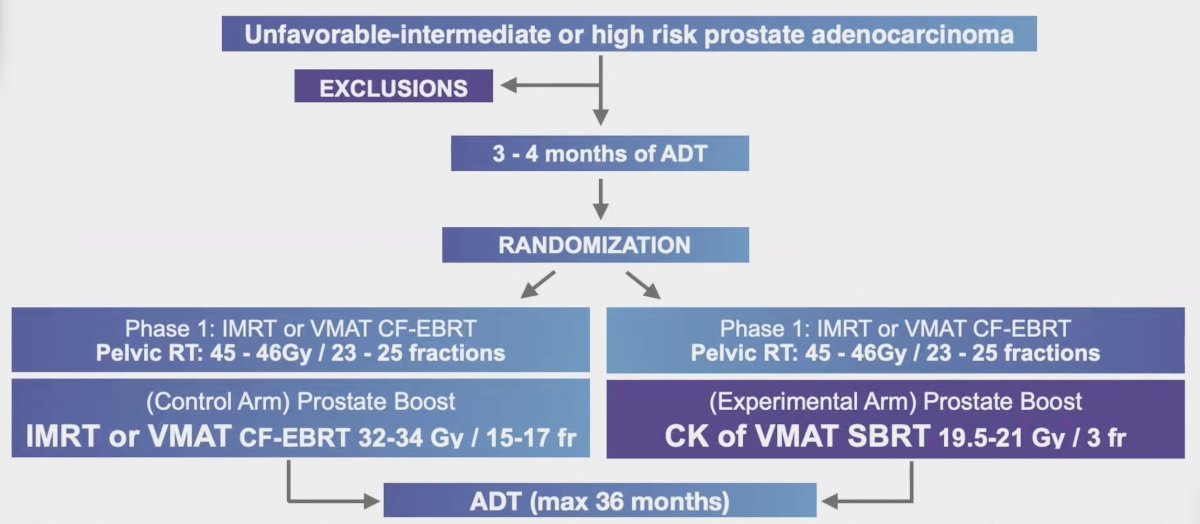
The primary objective was to assess early changes (3 months after radiotherapy) in gastrointestinal, genitourinary, and global patient-reported quality of life using the expanded prostate index composite (EPIC). Secondary outcomes included late genitourinary/gastrointestinal quality of life, IPSS scores, genitourinary/gastrointestinal CTCAE v.5.0 toxicity, and biochemical control up to 30 months.
Between September 10, 2019, and July 26, 2022, 53 patients were randomly assigned to conventional fractionation-external beam radiotherapy and 47 to stereotactic body radiotherapy boost radiotherapy. The baseline characteristics are as follows: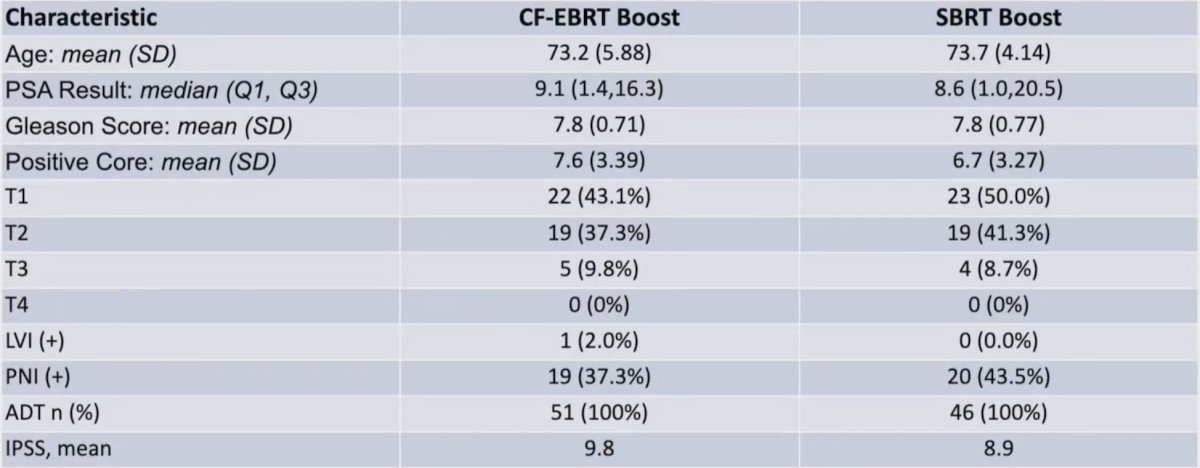
The mean follow-up time was 18.5 (range: 6-24) months. Comparing conventional fractionation-external beam radiotherapy with stereotactic body radiotherapy three months after the treatment, there were no significant differences in the mean urinary 11.5 versus 8.6, p = 0.233; bowel 5.2 versus 6.4, p = 0.573; and global 8.3 versus 7.5, p = 0.612, EPIC scores. Additionally, the changes in the IPSS scores were similar in both groups, with -7.0 versus -4.6, p = 0.105: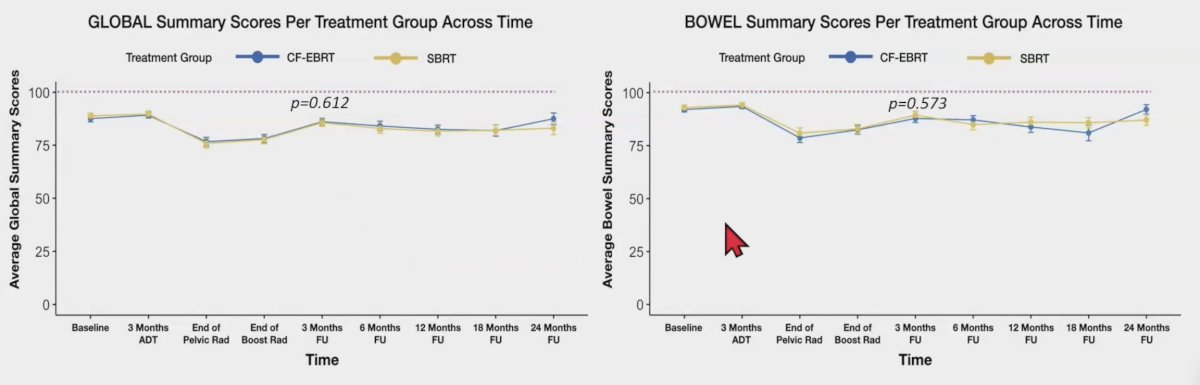
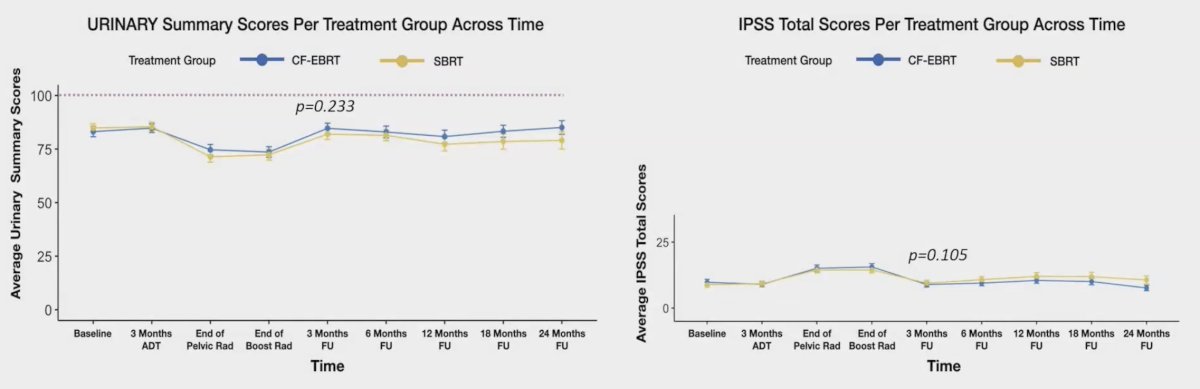
CTCAE v.5.0 gastrointestinal and urinary grade 2-4 toxicity rates after 3 months follow-up was comparable between the two groups, with an odds ratio of 0.90 (95% CI 0.22, 3.71). Rates of biochemical failure in both groups were <5% (very early).
Dr. Gouveia concluded his presentation discussing early results of PBS, a phase II trial assessing stereotactic body radiotherapy versus conventional fractionation external beam radiotherapy boost for unfavorable intermediate and high-risk prostate cancer with the following take-home points:
- In unfavorable intermediate and high-risk prostate cancer, comparison of stereotactic body radiotherapy boost treatment versus conventional fractionated-external beam radiotherapy of the prostate after conventional fractionation-external beam radiotherapy treatment of the pelvis demonstrated no significant differences in:
- EPIC quality of life scores (global, bowel, and urinary) at 3 months
- IPSS
- Toxicity rates (CTCAE v. 5.0)
- Early biochemical control rates appear similar
- Long-term quality of life, toxicity, and disease control results are awaited
Presented by: Andre Gouveia, MD, Clinical Fellow (Radiation Oncology), McMaster University, Hamilton, Ontario, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.


