(UroToday.com) The 2024 ASTRO annual meeting included a session on novel prognostic tools in prostate cancer, featuring a presentation by Dr. Yang Song discussing the association with a digital pathology multimodal artificial intelligence (MMAI) algorithm with pro-metastatic genomic pathways in oligometastatic prostate cancer.
Oligometastatic castration-sensitive prostate cancer is a heterogeneous disease. Recently, an MMAI algorithm (ArteraAI Prostate Test), which incorporates digital pathology and clinical information, has been shown to be prognostic in localized prostate cancer:1-2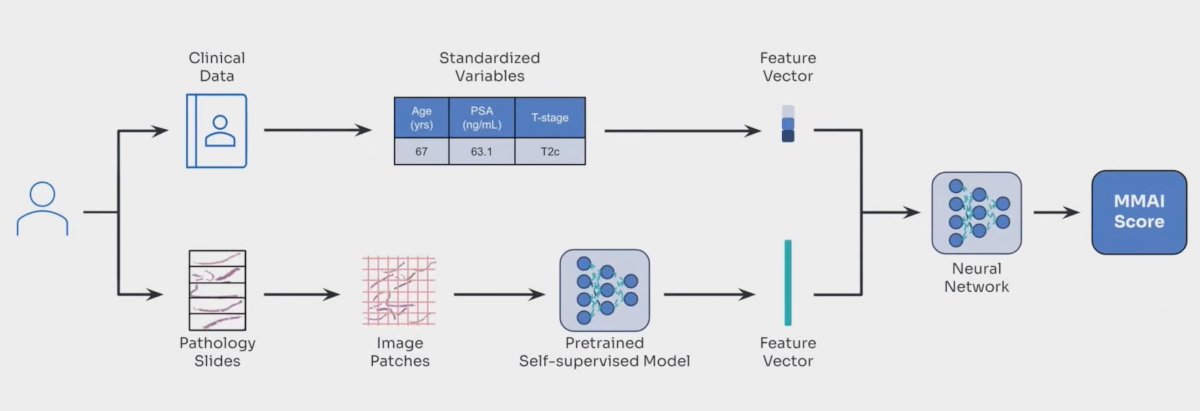
Presented at ASTRO 2024, Dr. Sutera and colleagues showed that among men with oligometastatic castration-sensitive prostate cancer, patients with a high MMAI had a significantly worse overall survival (HR 7.88, 95% CI 1.62-36.49) with a median overall survival of 108.4 months versus not reached:
Dr. Song notes that the MMAI scores is driven mostly by the artificial intelligence-detected digital pathologic features, however, what the artificial intelligence is “seeing” is not completely known: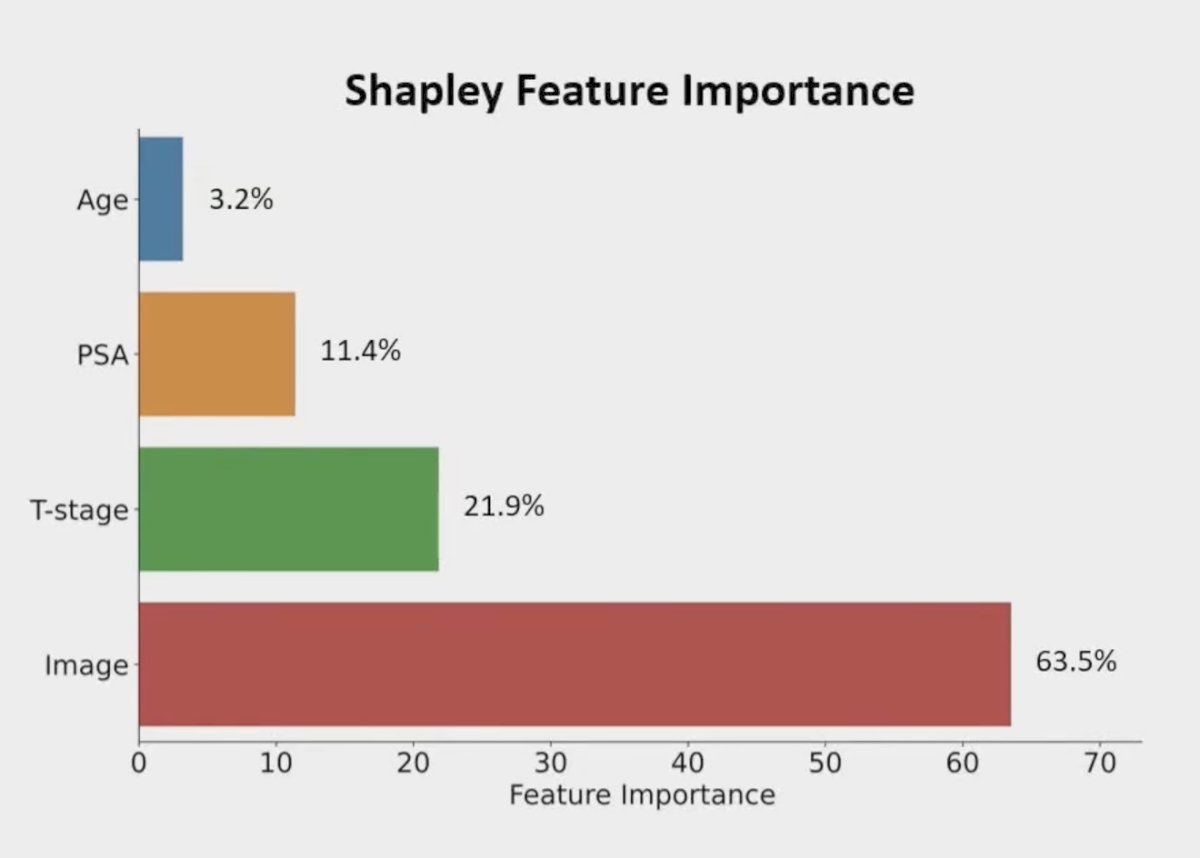
At the ASTRO 2024 annual meeting, Dr. Song and colleagues evaluated the association between the MMAI score and vector features of the score with genomics in oligometastatic castration-sensitive prostate cancer.
Dr. Song and investigators correlated somatic pathogenic mutations and ArteraAI MMAI scores from 168 oligometastatic castration-sensitive prostate cancer patients (133 metachronous and 35 synchronous). RNAseq profiling was performed on a subset of 65 metachronous patients, and somatic nonsynonymous pathogenic mutations from panel DNAseq were identified using Mutect2 and ClinVAR database. The consort diagram for this study is as follows: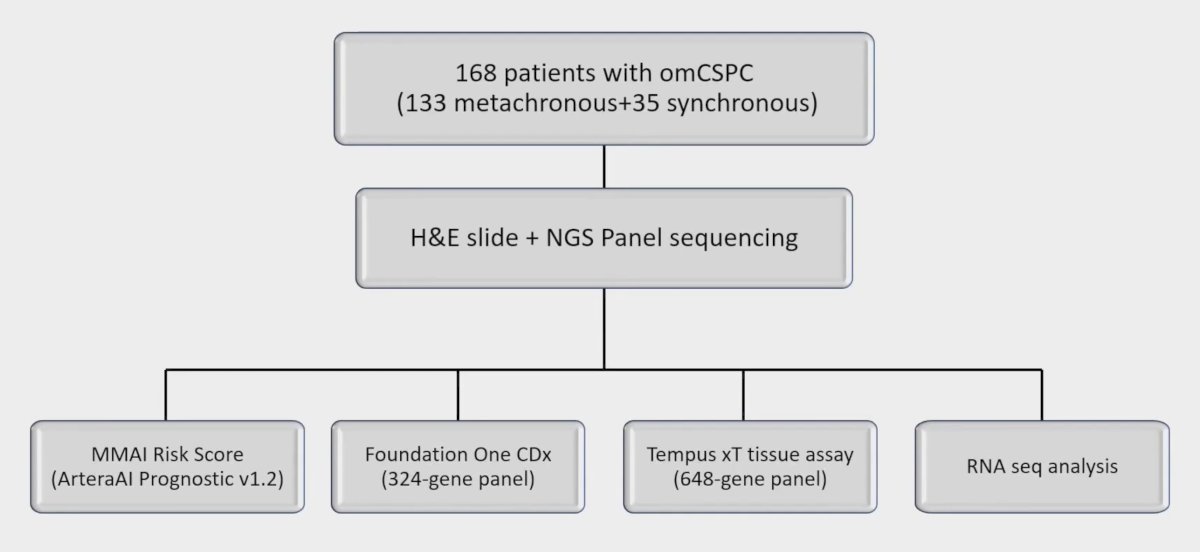
To identify the differentially mutated genes associated with patients with high/low MMAI scores, samples were binned separately based on the median or quartile of the MMAI score, and Fisher’s exact test was used to evaluate differentially mutated genes between bins. Differential expression of genes was evaluated using DEseq2 and gene set enrichment analysis. The median follow-up for this study was 34.7 months. Low MMAI (< median) score patients had more SPOP mutations, which are known to correlate with better prognosis:
The MMAI score also correlated with DNA damage response mutations (ie. BRCA2/ATM), with a higher frequency of DDR mutation observed in patients with higher MMAI scores (Q1-3 vs Q4, p = 0.34):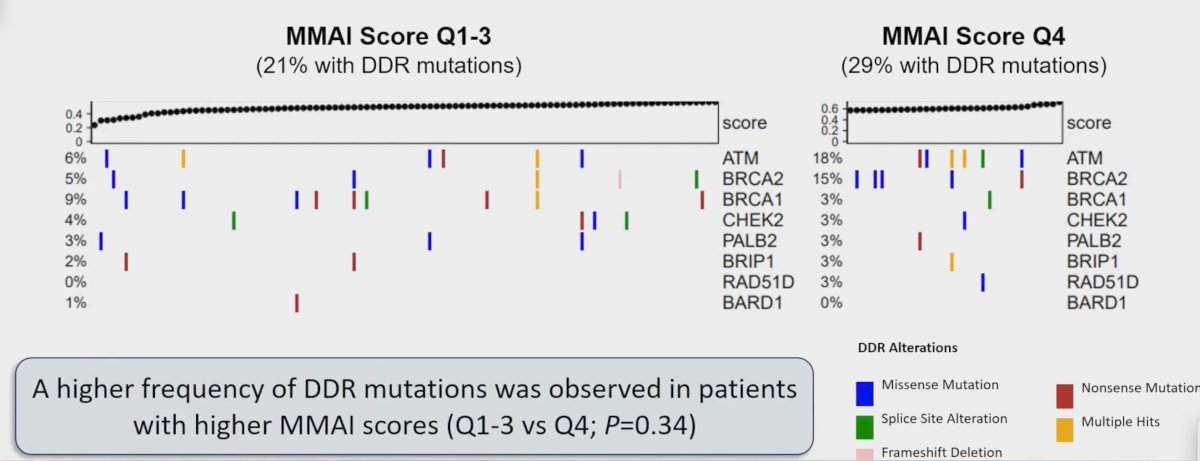
Moreover, DNAseq showed that high MMAI score (top median or quartile) had significantly more BRCA2/ATM mutations than those with low MMAI scores (p < 0.05):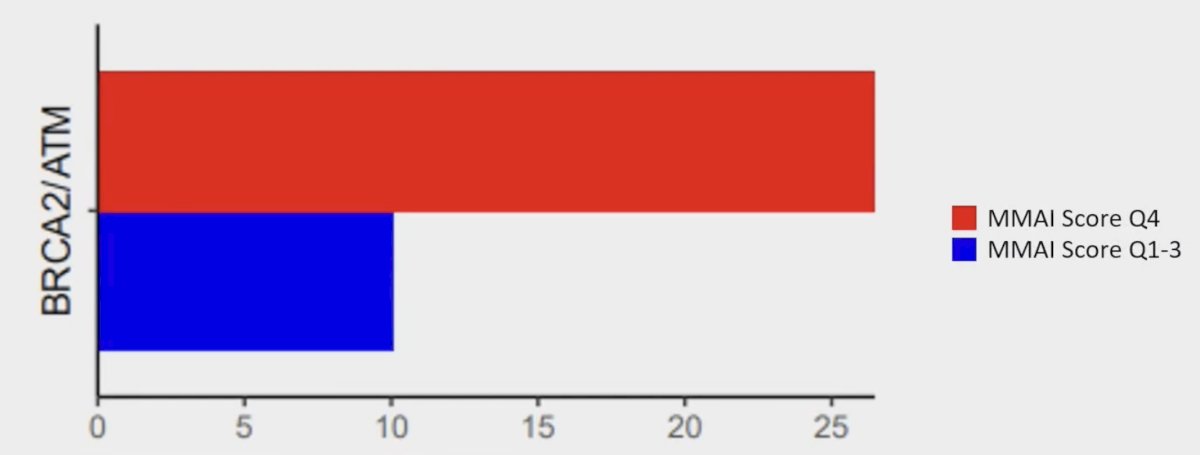
Similarly, DNAseq showed that high MMAI score (top median or quartile) had a trend towards more WNT pathway (APC/CTNNB1) mutations (p = 0.13):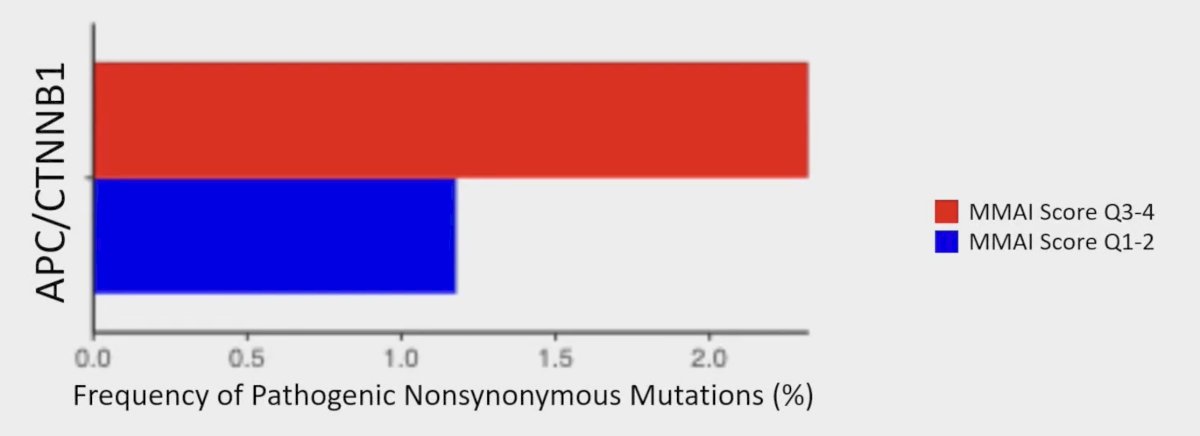
Finally, RNAseq showed differentially expressed genes of patients with high MMAI scores were enriched for the epithelial-mesenchymal transition pathway (p < 0.01):
Dr. Song concluded her presentation discussing the association with a digital pathology multimodal artificial intelligence algorithm with pro-metastatic genomic pathways in oligometastatic prostate cancer with the following take-home points:
- A digital pathology-based MMAI biomarker was prognostic for overall survival among patients with oligometastatic castration-sensitive prostate cancer
- Mutations in genes in the pro-metastatic DDR pathway (ie. BRCA2/ATM) were statistically correlated with increasing MMAI scores
- Higher MMAI scores trended towards more WNT pathway mutations (ie. APC/CTNNB1)
- MMAI scores were negatively associated with SPOP mutation frequency in metachronous oligometastatic castration-sensitive prostate cancer
- MMAI scores correlated with an epithelial-mesenchymal transition pathway at the transcriptome level
Presented by: Yang Song, Research Scientist, University of Maryland, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.
Related content: MMAI Score Prognostic for Overall Survival in Oligometastatic Castration-Sensitive Prostate Cancer - Phuoc Tran & Tim Showalter
References:
- Esteva A, Feng J, van der Wal D, et al. Prostate cancer therapy personalization via multi-modal deep learning on randomized phase III clinical trials. NPJ Digit Med. 2022 Jun 8;5(1):71.
- Spratt DE, Tang S, Sun Y, et al. Artificial Intelligence Predictive Model for Hormone Therapy Use in Prostate Cancer. NEJM Evid 2023;2(8).


