Based on these results, CG0070 was taken to a phase II open label trial. Initial, early results were published last year by Packiam et. al.2 Patients who were BCG unresponsive and refusing cystectomy were eligible for the study.
They have previously demonstrated promising 6 month response using intravesical CG0070, but longer term efficacy is unknown. At that time (April 2017), 45 patients with residual high-grade Ta, T1, or carcinoma-in-situ (CIS) ± Ta/T1 had 6 months follow-up. Complete response, defined as “as absence of disease on cytology, cystoscopy, and random biopsies,” was the primary outcome. They identified a 47% CR rate at 6 months for all patients and 50% for patients with CIS, with an acceptable level of toxicity for patients with high-risk BCG-unresponsive NMIBC. It should be noted that no patient with pure T1 disease had 6-month CR.
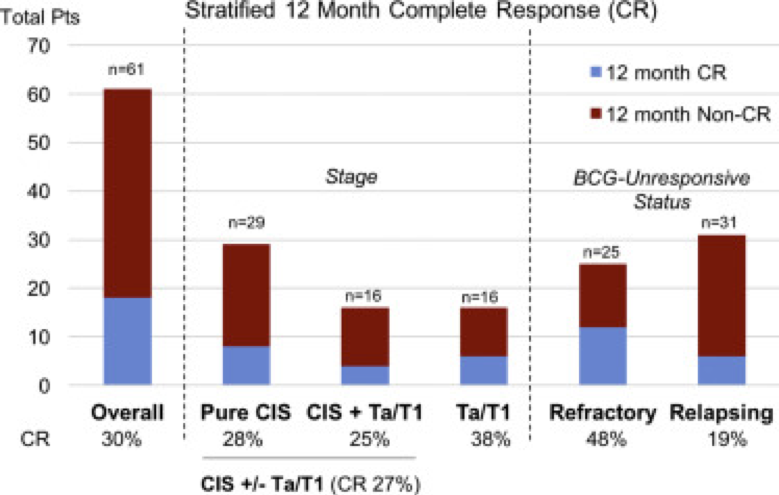
They now present a 12-month interim analysis. They are now up to 67 patients – of which, 6 were excluded due to going on to subsequent therapy despite having CR prior to 12 months and not having 12 months follow-up yet. As such, they had 61 patients in the cohort. Patients were 72 years old on average and predominantly male (81%).
All these patients were BCG unresponsive per the new definition – and included BCG refractory (25 patients) and BCG relapsing (31) patients. Most had CIS – either pure CIS or in conjunction with papillary diease. They received weekly instillations of CG0070 for 6 weeks (induction), and then again at 6, 12, and 18 month maintenance time points.
Overall 12 month CR rate was 30%. CIS-containing tumors had 27% 12 month CR (n=45) while pure Ta/T1 had 38% (n=16). No patients with T1/CIS or Ta/CIS had 12 month CR (n=6). When looking at BCG response: BCG-refractory patients had 48% (n=25) 12 month CR, BCG-relapsed 19% (n=31), unknown 0% (n=5). Following trial entry, 10 patients underwent cystectomy, of which 6 patients had muscle invasive disease.
In terms of tolerability, all 12 month treatment related adverse events (AEs) were Grade 1-3; immunologic AEs included influenza type illness (7%), fatigue (4%), and chills (1%). Five deaths were secondary to progressive urothelial carcinoma, esophageal carcinoma, lung carcinoma, and cardiac disease.
Overall, tolerability remained quite good. In general, the intervention appears to be less effective in CIS patients. The summary of response rates stratified by initial histology and BCG response were presented in the following table:
References:
1. Burke JM, Lamm DL, Meng MV, Nemunaitis JJ, Stephenson JJ, Arseneau JC, Aimi J, Lerner S, Yeung AW, Kazarian T, Maslyar DJ, McKiernan JM. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012 Dec;188(6):2391-7. doi: 10.1016/j.juro.2012.07.097. Epub 2012 Oct 22.
2. Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL 3rd, Clark W, Kroeger M, Dumbadze I, Chamie K, Kader AK, Curran D, Gutheil J, Kuan A, Yeung AW, Steinberg GD. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. 2017 Jul 26. pii: S1078-1439(17)30350-2. doi: 10.1016/j.urolonc.2017.07.005. [Epub ahead of print]
Presented by: Vignesh T Packiam, MD. University of Chicago
Co-Authors: Daniel A Barocas, Karim Chamie, Ronald L Davis III, A Karim Kader, Donald L Lamm, John Gutheil, Arthur Kuan, Gary D Steinberg
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, @tchandra_uromd at the 2018 AUA Annual Meeting - May 18 - 21, 2018 – San Francisco, CA USA


