Another PET radiotracer is Choline, which is used in the setting of biochemical recurrence. This has been FDA approved since 2011. Choline is a component of cell membranes. Despite having good specificity and sensitivity, there is limited detection sensitivity when PSA < 2 ng/ml.
FACBC-Fluciclovine is taken up by two amino acid transporters that are upregulated in prostate cancer cells. It has been shown to outperform C11 Choline, and has been FDA approved since June 2016 (Figure 1).
Figure 1:
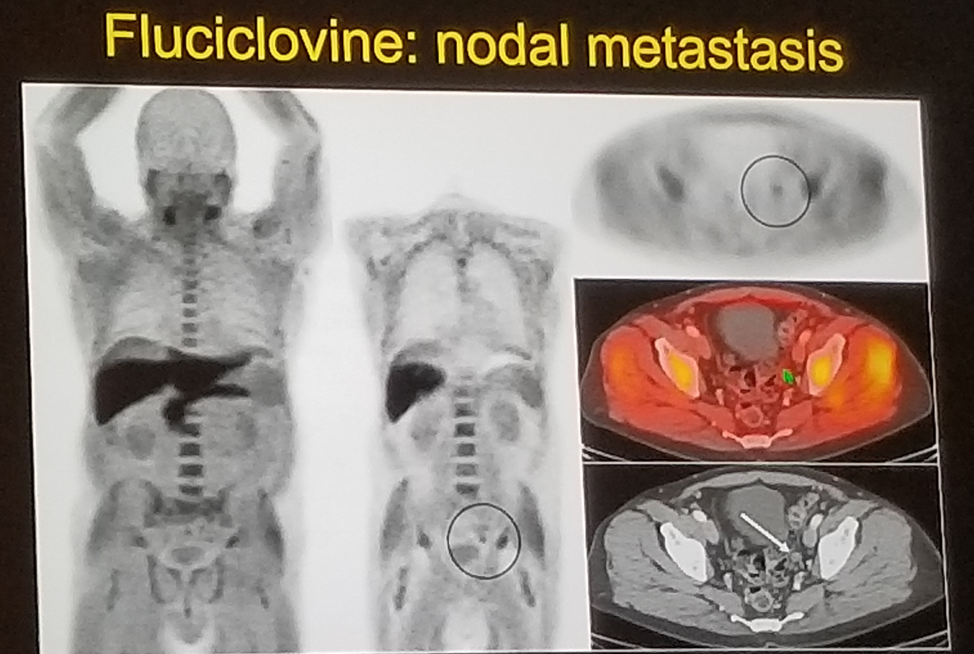
Ga-68 PSMA-11 prostate specific membrane antigen is probably the best PET modality to date for prostate cancer. It binds the extracellular domain (as opposed to ProstaScint that was bound to the intracellular domain). It has been demonstrated to have a higher sensitivity than fluorocholine in patients with biochemical recurrence. PSMA is currently being used worldwide with more and more countries using it for different stages of prostate cancer disease.
Lastly, F-DCFPyL, which is termed the PyL compound, has low blood pool activity and is currently in phase 2/3 trials.
In summary, there are a growing number of radiotracers. Fluciclovine is FDA approved, widely available and reimbursed by Medicare. PSMA PET has a higher detection sensitivity but is limited to the research setting in the USA.
Presented by: Thomas Hope, MD, Chief of MRI and Nuclear Medicine, San Francisco VA Medical Center
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 AUA Annual Meeting - May 18 - 21, 2018 – San Francisco, CA USA


