A total of 1207 pts (median age, both arms: 74 y) with nmCRPC and PSA doubling time of 10 mo were randomized 2:1 to APA (240 mg QD) or PBO. ADT was continued. HRQoL and symptoms were assessed using PRO questionnaires, including the Functional Assessment of Cancer Therapy-Prostate (FACT-P), Euro QoL Group EQ-5D3L. PROs were completed at baseline, Day 1 of Cycles (C) 2-7, C9, C11, C13, and then every 4 mo for the duration of treatment and 1 y after treatment discontinuation. Analyses were conducted on FACT-P total score and all subscales, including FACT-G, a more general evaluation of HRQoL.
The compliance rate for completion of FACT-P and EQ5D at all treatment phase assessment visits was 92% (range 92-100%). Group means at baseline for FACT-G (APA, 83; PBO, 84; out of a total score of 108) and were consistent with the FACT-G population norm of (mean: 81) for adult men. Median treatment exposure was: APA, 16.9 mo; PBO, 11.2 mo. Mean change from baseline in FACT-P total and subscales was generally not significantly different over time comparing APA vs PBO. Any significant differences represented better scores for APA. No significant differences were observed comparing APA vs PBO in time to degradation analyses in FACT-P total and subscale scores. Overall, FACT-G and other PRO scores were maintained with APA and ADT from initiation of treatment (Figure below).
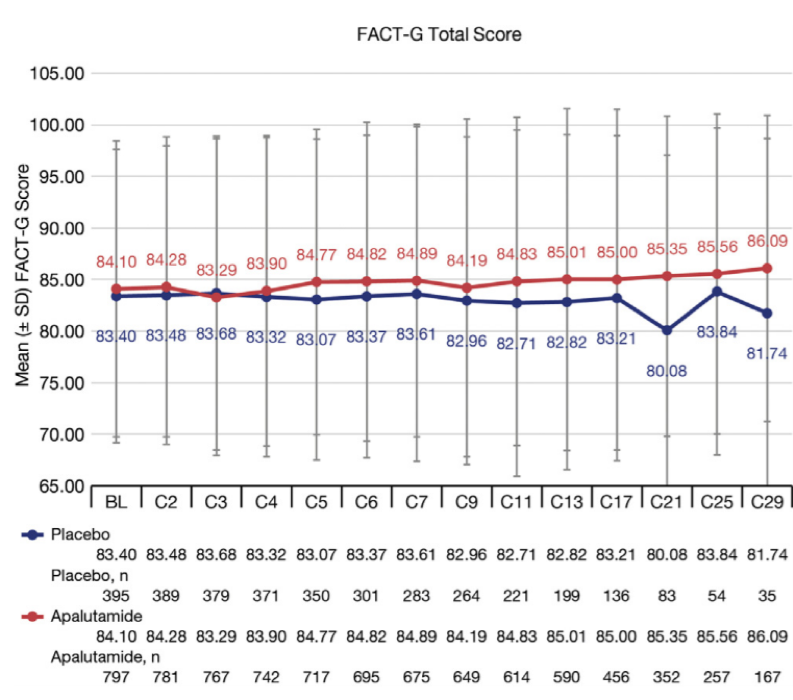
In conclusion, baseline HRQoL data suggest that men with nmCRPC have similar HRQoL to the general population. The addition of APA to ADT maintained HRQoL and significantly improved MFS in this patient population, who are largely asymptomatic at baseline.
Presented by: Eric J. Small, MD, UCSF Helen Diller Family Comprehensive Cancer Center
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2018 AUA Annual Meeting - May 18 - 21, 2018 – San Francisco, CA USA
Further Related Content:
Eric J. Small, MD 2018 AUA Presentation Results from SPARTAN: PSA Outcomes in Patients with Nonmetastatic Castration-Resistant Prostate Cancer Treated with Apalutamide
Watch: First presentation of SPARTAN - a phase 3 double-blind, randomized study of apalutamide (APA) versus placebo in patients with nonmetastatic castration-resistant prostate cancer (nmCRPC) - Eric Small
Watch: M0 CRPC Outcomes in the Use of New Androgen Receptor-Targeted Therapies - A Conversation with Charles Ryan and Alicia Morgans
Watch: The Incredible Shrinking M0 CRPC - Phillip Koo


