Dr. Chang presented a clinical trial which involves the use of a novel immunotherapy intervention with first-in-class Igg-1-Fc IL-15 cytokine agonist (N-803). The idea was to give this novel agent together with BCG to patients with recurrence or relapse of a disease following intravesical BCG therapy. N-803 has a unique mechanism of action, as seen in figure 1. IL-15 signaling is performed via trans-presentation with IL-15R alpha selectively inducing CD8+ and NK cells, as seen in figure 2. The N-803 mimics trans-presentation of IL-15
Figure 1—The mechanism of action of N-803:
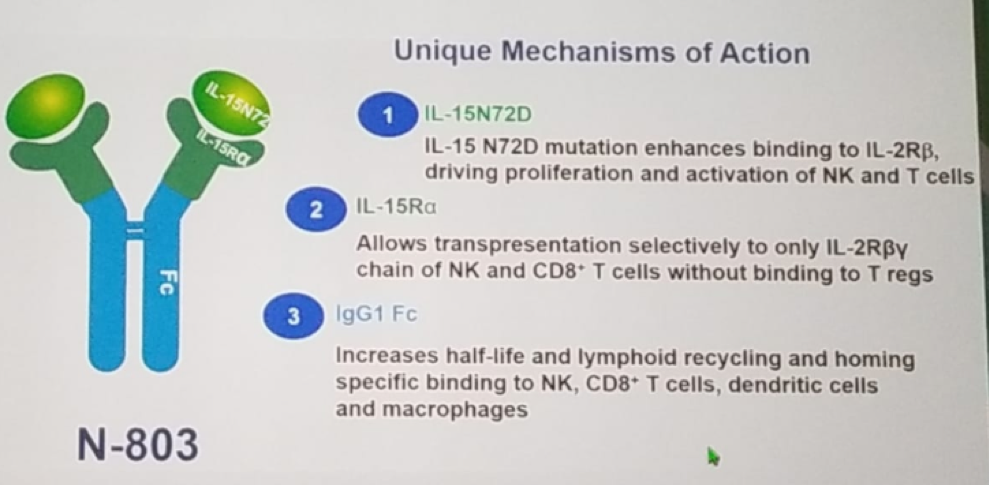
Figure 2 – IL-15 signaling:

BCG uptake causes bladder cancer cells to present antigen and to produce immune stimulating cytokines. The cytokines and molecular stress signals recruit immune effector cells, which attack cancer. However, in BCG unresponsive disease, BCG may be unable to trigger a strong enough antitumor immune response to clear cancer. However, with proper immunes stimulation, it may be possible to rescue immune response to BCG.
In a rat model, it has been shown that a combination of BCG and N-803 have reduced the tumor burden. In the phase 1 trial, N-803 +BCG were given to high-risk NMIBC patients. Dose escalations were performed to determine the recommended dose. The adverse events included hypertension, hematuria, and pollakiuria. These adverse effects were similar in severity and duration to the historical treatment with BCG alone.
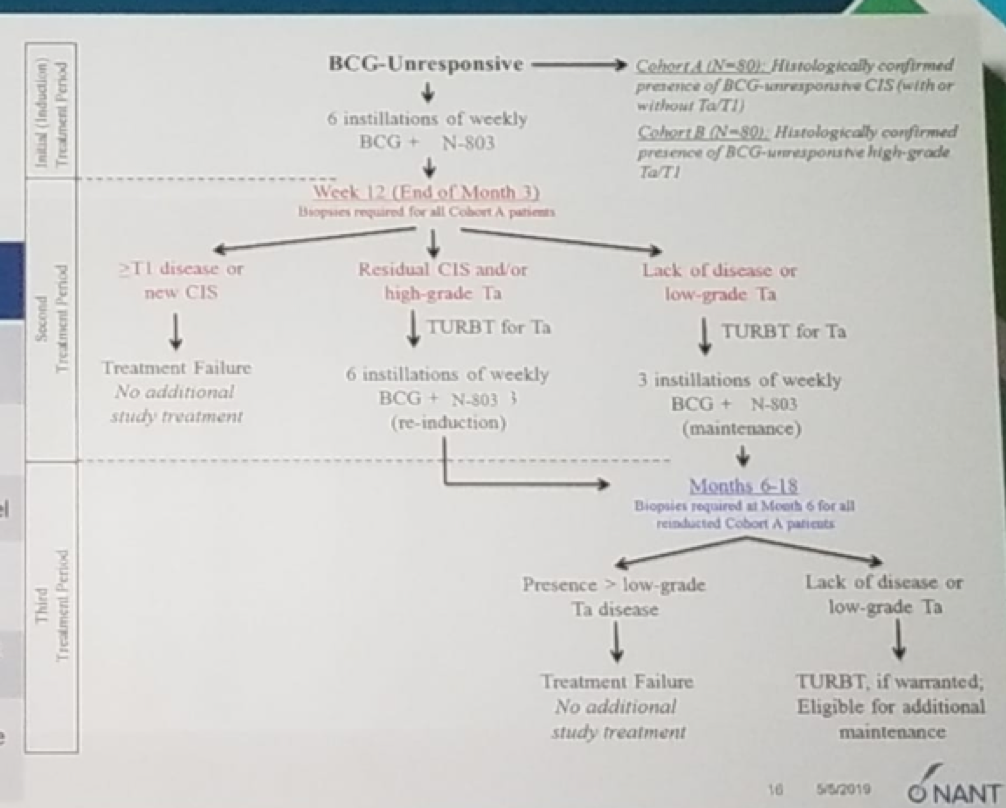
In the currently presented single-arm, open-label phase two trial, 160 patients (80 had CIS, and 80 had a papillary tumor) with BCG-unresponsive NMIBC were accrued. All patients received intravesical N-803 and BCG. The primary endpoint was a complete response at any time with CIS (for the CIS patients), and 12-month disease-free survival (For the papillary tumor patients). The trial design is shown in figure 3.
Up to now, in the CIS cohort, 35 patients have been enrolled, with 16 patients experiencing complete response out of 18 evaluable patients (89% complete response rate). In the papillary cohort, 27 patients have been enrolled, and the six-month recurrence-free survival rate is 77% (10 out of 13 patients). With regards to safety, the adverse events that were encountered seemed to be similar to those associated with BCG. There have been to date only five adverse events (2 in the CIS cohort, and 3 in the papillary cohort). All of the adverse events were determined to be unlikely related to the combination of N-803 + BCG.
Dr. Chang summarized the results and stated that phase 1 and phase 2 studies have shown that this combination treatment has a similar safety profile as BCG alone. Early efficacy in the CIS cohort shows a high complete response rate of 89% at any time. In the papillary cohort, the 6-month recurrence-free survival was 77%. In conclusion, this combination treatment seems to be well-tolerated, produce a complete response rate in a significant number of CIS patients, and reduce recurrence in a significant number of patients with Ta/T1 disease. Longer follow-up is still required to determine the ultimate success of this combination therapy.
Figure 3 – Trial design:
Presented by: Sam S. Chang, MD, MBA, Patricia and Rodes Hart Endowed Professor of Urologic Surgery and Oncology, is the Oncology Fellowship Director and Vice-Chair of Urologic Surgery at Vanderbilt University Medical Center.
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the American Urological Association's 2019 Annual Meeting (AUA 2019), May 3 – 6, 2019 in Chicago, Illinois
References:
- Babjuk M et al. EAU guidelines 2015
- Chang SS et al. AUA guidelines 2016


