High-grade disease is known to be an adverse prognostic feature in UTUC. The POUT trial1 has demonstrated that adjuvant chemotherapy improves disease-free survival in UTUC patients (figure 1).
Figure 1 – POUT trial results:

In this multicenter prospective single-arm phase two trial (NCT01261728), the benefit of neoadjuvant chemotherapy in UTUC was assessed. The key eligibility criteria are shown in Figure 1. The study design is shown in figure 2. All patients underwent either radical nephroureterectomy or distal ureterectomy following neoadjuvant chemotherapy. The surgery also entailed ipsilateral retroperitoneal lymph node dissection using a standardized template (Figure 3). The follow-up protocol was quite standard in these patients, as shown in table 1. The primary endpoint was pathological response rate defined as <=pT1N0. The secondary objectives included time to disease progression, overall survival, safety, and tolerability. The accrual goal was 54 patients, with a minimum of 28 responders required. The patient disposition is shown in figure 4.
Figure 1- Key eligibility criteria:

Figure 2- Study design:
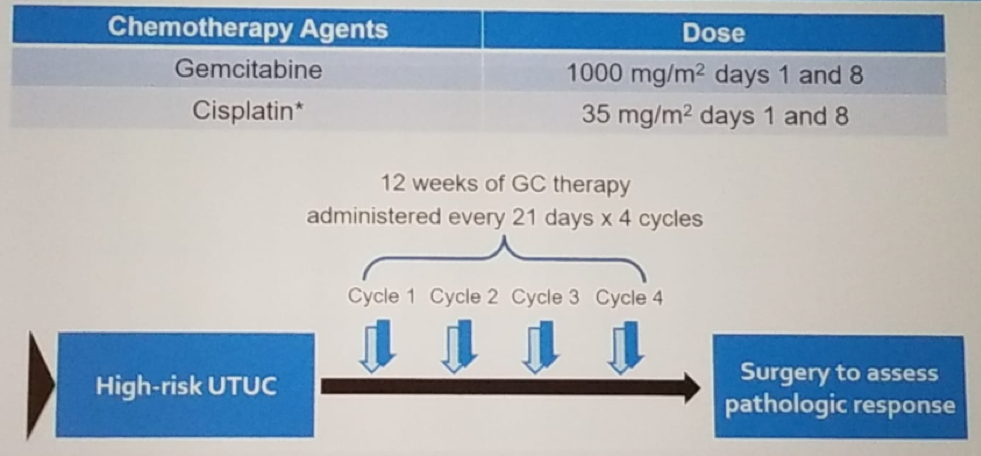
Figure 3 – Ipsilateral retroperitoneal lymph node dissection template:
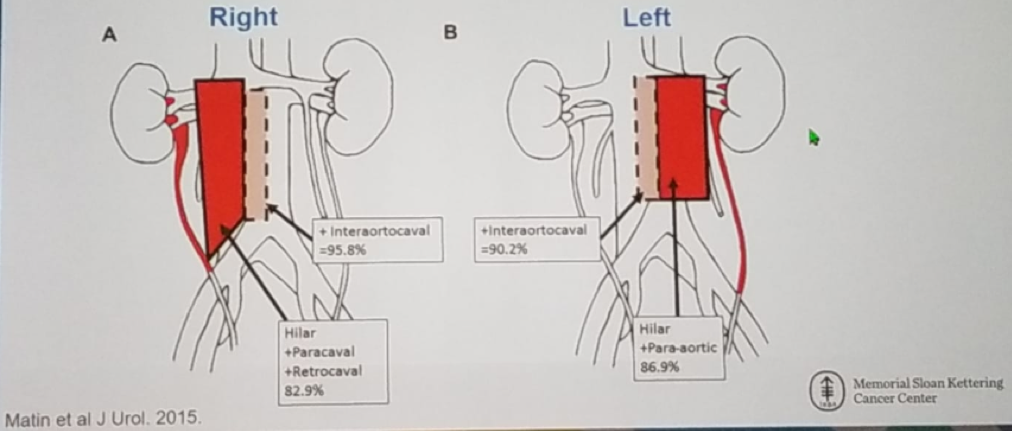
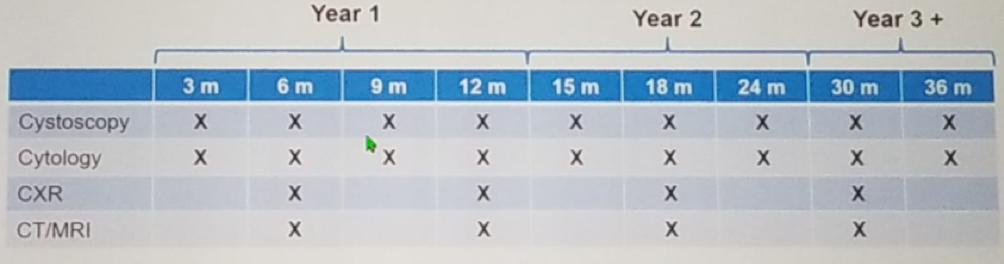
Figure 4 – Patient disposition:
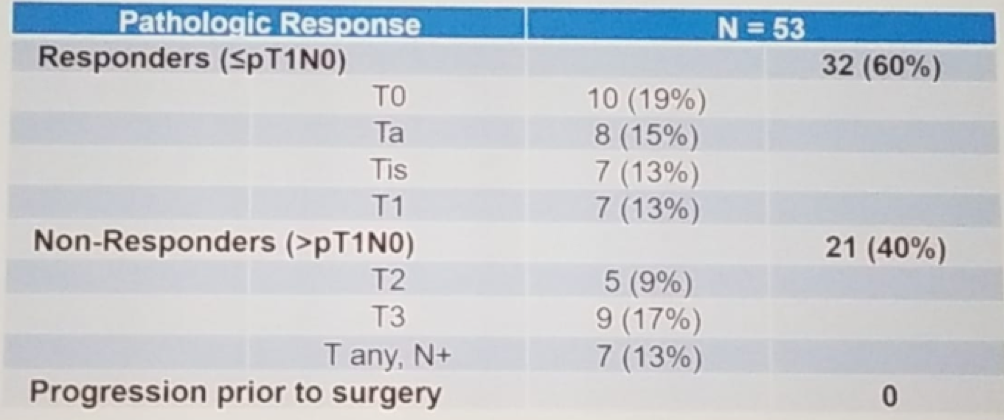
The pathologic response rate was 60% (95% CI 47%-75%), as seen in table 2. The two-year progression-free survival was 76% (95% CI 64%-90%) (Figure 5), with a median follow-up for survivors of 2.6 years. The two-year overall survival was 89% (95% CI 80-100%), and an improved progression-free survived (Figure 5). Lastly, the chemotherapy adverse -related toxicities include leukopenia (72%), neutropenia (64%), thrombocytopenia (66%), rise in creatinine (40%), and thromboembolism in 17%. The split dose of gemcitabine and cisplatin was well -tolerated. The perioperative outcomes did not appear to be adversely affected, as seen in table 3.
Table 2 – Pathologic response rate:


Figure 5: Progression free survival:
Dr. Wong concluded his extensive talk, stating that neoadjuvant chemotherapy in this trial met the primary endpoint with 60% response rate. Neoadjuvant chemotherapy followed by radical nephroureterectomy and ipsilateral templated retroperitoneal lymph node dissection achieved a two-year progression-free survival of 75% and two-year overall survival of 89%. This approach of neoadjuvant chemotherapy enables more UTUC patients the ability to benefit from the combined modality therapy. According to Dr. Wong, the combined modality of neoadjuvant chemotherapy and definitive surgery with lymph node dissection should be considered a standard of care option for high-grade UTUC.
Presented by: Nathan Wong, BSc (H), MD FRCSC, Urologic Oncology Fellow at Memorial Sloan Kettering Cancer Center, Memorial Sloan Kettering Cancer Center
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the American Urological Association's 2019 Annual Meeting (AUA 2019), May 3 – 6, 2019 in Chicago, Illinois
- Birtle, A. (2018). ASCO GU 2018: Results of POUT: A Phase III Randomized Trial of Perioperative Chemotherapy versus Surveillance in Upper Tract Urothelial Carcinoma (UTUC). [online] Urotoday.com. [Accessed 5 May 2019].
- Lame et al. Cancer 2010
- Xylinas et al. BJUI 2013
- Grossman et al. "Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer".NEJM 349 (2003):859-866
DOI: 10.1056/NEJMoa022148 - Hussain et al. "A phase I/II study of gemcitabine and fractionated cisplatin in an outpatient setting using a 21-day schedule in patients with advanced and metastatic bladder cancer". Br J Cancer, 91(5) (Aug 2004):844–849


