From a historical perspective, a seminal observation that prostate cancer is an androgen driven/dependent disease and surgical or medical castration can induce significant regressions of prostate cancer was made. Although >90% of patients initially respond to ADT, however, most will progress to castration resistance with a median survival of about 4 years. Dr. Hussain outlines that there have been several treatment strategies tested over the past 7 decades for patients with metastatic hormone-sensitive prostate cancer:
- Bilateral orchiectomy vs medical castration
- Gonadal suppression vs peripheral blockade
- LHRH agonist vs antagonist
- Combined androgen deprivation vs gonadal suppression
- ADT +/- bone targeted therapy
- Intermittent ADT vs continuous ADT
As follows are the pathways/targets and agents involved and/or being evaluated:
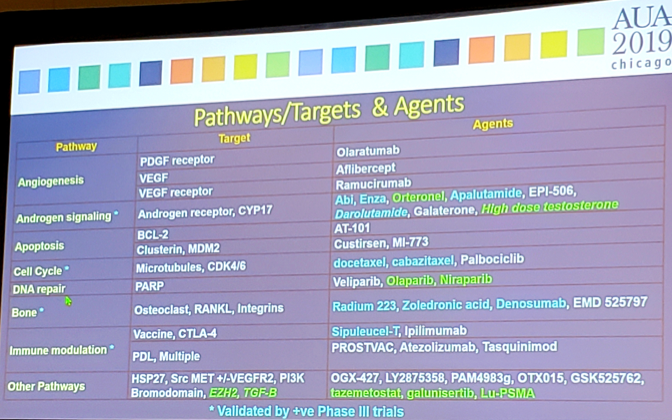
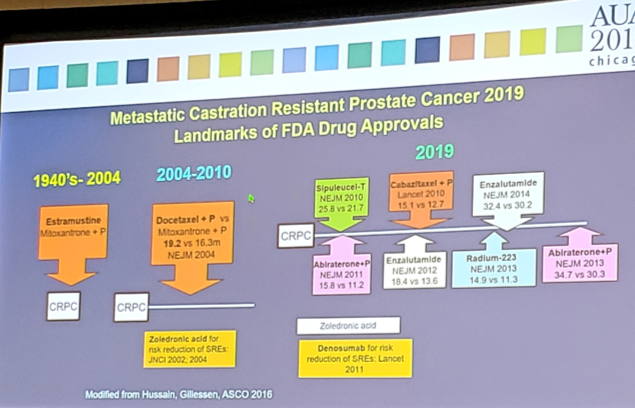
Dr. Hussain notes that there have been several revelations over the last 10-15 years for men with advanced hormone naïve prostate cancer.
#1 Earlier is Better: Chemohormonal Therapy for Metastatic Hormone Sensitive Prostate Cancer
In 2015, the CHAARTED trial changed the landscape of treatment of men with mHSPC. This trial randomized 790 men with mHSPC to receive either ADT + docetaxel (75 mg/m2 every 3 weeks for six cycles) (ADT-DOCE) or ADT alone, with OS as an endpoint [2]. After a median follow-up of 28.9 months, the median overall survival was 13.6 months longer with ADT-DOCE than with ADT alone (57.6 months vs. 44.0 months; HR 0.61; 95%CI 0.47-0.80). Furthermore, the median time to biochemical, symptomatic, or radiographic progression was 20.2 months in the ADT-DOCE group, as compared with 11.7 months in the ADT-alone group (HR 0.61, 95%CI 0.51-0.72). This trial ushered into clinical practice ADT-DOCE as standard of care for men with mHSPC. In 2018, the CHAARTED trial published an updated survival analysis: at a median follow-up 53.7 months, the HR for OS was 0.72 (95%CI 0.59-0.89) favoring docetaxel over ADT standard of care, a 28% risk reduction of death compared to 39% in the first analysis [3]. In subset analyses, the benefit was observed across all subgroups with two notable exceptions. Specifically, patients with low burden of disease (HR 1.04, 95%CI 0.7-1.55) or those who had prior local therapy (HR 0.97, 95%CI 0.58-1.56) did not seem to experience a benefit through the addition of docetaxel to standard ADT.
#2 Earlier is Better: Abiraterone/prednisone + ADT in newly diagnosed high-risk metastatic hormone-naïve prostate cancer
LATITUDE was an international trial evaluating ADT-ABI compared to ADT alone among men with high-risk mHSPC [4]. High-risk was defined as meeting at least two of three criteria: (i) Gleason score ≥8, (ii) presence of ≥3 lesions on bone scan, or (iii) presence of measurable visceral lesions. Patients were randomized 1:1 to either ADT-ABI (1000 mg abiraterone acetate + 5mg prednisone daily) (n=597) or ADT + placebo (n=602). The co-primary endpoints were OS and radiographic progression-free survival (rPFS). Secondary endpoints included time to: pain progression, PSA progression, next symptomatic skeletal event, chemotherapy, and subsequent prostate cancer therapy. Over a median follow-up of 30.4 months, patients treated with ADT-ABI had a 38% risk reduction of death (HR 0.62, 95%CI 0.51-0.76) compared to ADT + placebo. Median OS was not yet reached in the ADT-ABI arm, compared to 34.7 months in the ADT + placebo arm. There was also a 53% risk of reduction of radiographic progression or death for patients treated with ADT-ABI compared to ADT alone (HR 0.47, 95%CI 0.39-0.55). Additionally, there was a statistically significant improvement across all secondary endpoints for ADT-ABI:
- Time to PSA progression (HR 0.30, 95%CI 0.26-0.35)
- Time to pain progression (HR 0.70, 95%CI 0.58-0.83)
- Time to next symptomatic skeletal event (HR 0.70, 95%CI 0.54-0.92)
- Time to chemotherapy (HR 0.44, 95%CI 0.35-0.56)
- Time to subsequent prostate cancer therapy (HR 0.42, 95%CI 0.35-0.50)
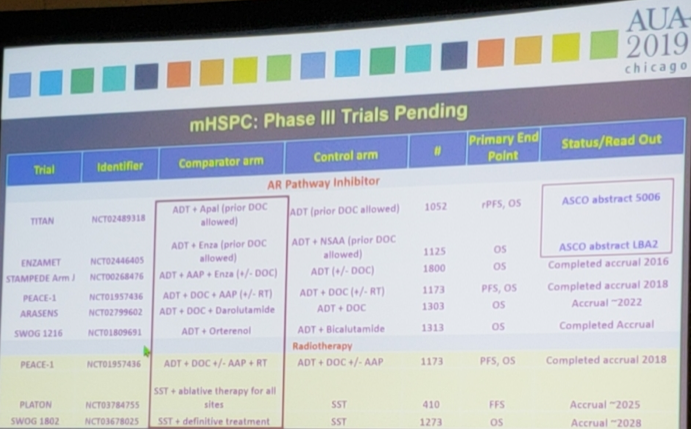
There are several ongoing clinical trials in the metastatic hormone naïve disease space, two of which will be reporting outcomes at the 2019 ASCO meeting:
Dr. Hussain poses the question: So why is time for genomics? Prostate cancer is a very complex process, as there are large clonal alterations plus inter-tumoral heterogeneity in different metastatic sites. Approximately 90% of mCRPC patients harbor clinically potentially actionable molecular alterations including PIK3CA/B, R-spondin, BRAF/RAF1, APC, Beta-catenin and ZBTB16/PLZF. Furthermore, more than 20% of mCRPC patients harbor DNA repair pathway alterations (BRCA2, BRCA1, ATM and others).
Dr. Hussain concluded her talk with an overarching vision – Conquering cancer through research and excellence in care:
- Better imaging – identifying a window of opportunity
- Maximizing the antitumor effect: finding novel pathways and agents, understanding mechanisms of resistance, and finding multi-targeted strategies
- Personalizing therapy and precision medicine
- Enhancing survivorship and addressing cost/value
Presented by: Maha Hussain, MD, FACP, FASCO, is the Genevieve Teuton Professor of Medicine in the Division of Hematology Oncology, Department of Medicine, and the Deputy Director at the Robert H. Lurie Comprehensive Cancer Center of the Northwestern University Feinberg School of Medicine, Chicago, IL
Written By: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University - Medical College of Georgia, Twitter: @zklaassen_md at American Urological Association's 2019 Annual Meeting (AUA 2019), May 3 – 6, 2019 in Chicago, Illinois
References:
Further Related Content:
Watch: Life-Prolonging Treatments for Advanced Prostate Cancer - Maha Hussain
- Hussain M, Goldman B, Tangen C, et al. Prostate cancer antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol 2009 May 20;27(15):2450-2456.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
- Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36:1080-7.
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377:352-60.
- Fizazi K, Feyerabend S, Matsubara N, Ozguroglu M, Fein L, Rodriguez-Antolin A, et al. Longer term preplanned efficacy and safety analysis of abiraterone acetate + prednisone (AA + P) in patients (pts) with newly diagnosed high-risk metastatic castration-naïve prostate cancer (NDx-HR mCNPC) from the phase 3 LATITUDE trial. J Clin Oncol. 2018;36:Abstr 5023.
Further Related Content:
Watch: Life-Prolonging Treatments for Advanced Prostate Cancer - Maha Hussain


