This study was an open pilot randomized clinical trial 1:1 including patients with high-risk non-muscle-invasive bladder cancer according to EAU Guidelines.2 Patients with carcinoma in situ (CIS), intolerance or contraindication for receiving BCG or mitomycin C were excluded.
Patients were randomly assigned to one of the following groups: (1) BCG (TICE strain): 50 mg diluted in 50 mL of sterile saline held for 2 hours in the bladder, one weekly instillation for 6 weeks and maintenance according to SWOG protocol; (2) Chemohyperthermia: 40 mg mitomycin C diluted in 40 mL of distilled water at 43ºC using COMBAT® recirculation system for 60 minutes, one weekly instillation for 6 weeks and one monthly instillation for 6 months. The outline for this trial is as follows:
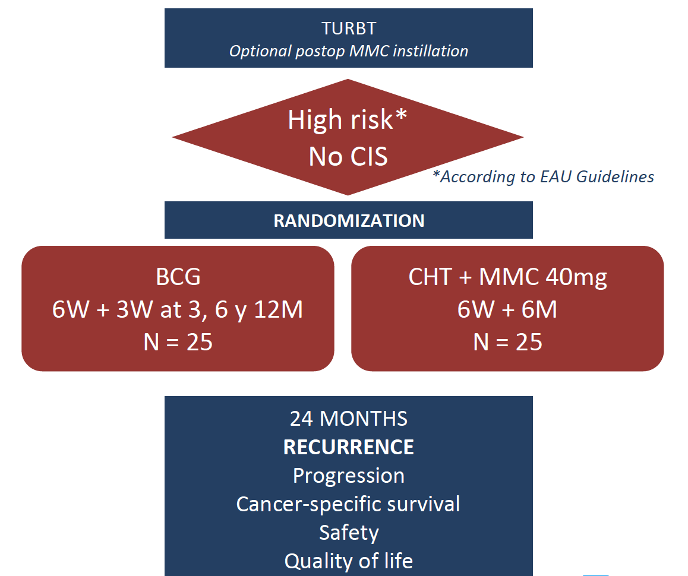
Follow-up was performed with cytology and cystoscopy every 3 months as well as upper urinary tract imaging yearly, as stated by European Association of Urology (EAU) Guidelines. The primary endpoint was recurrence-free survival at 24 months, and secondary objectives included safety, progression rate, overall survival, and quality of life.
There were 50 patients randomized (100% recruitment completed), and 48 started treatment. The median age was 73 years, 87.6% were males and 83% had primary tumors; baseline characteristics were comparable in both groups. The median follow-up to date was 24.8 months from TURBT: for the BCG group, 6 recurrences (from which five patients progressed to T2) were reported, and only 3 recurrences (from which two patients progressed to T2) happened in the chemohyperthermia group. Regarding the safety profile, adverse events appeared in 12 patients from the chemohyperthermia group and 10 from the BCG group, with no differences in the severity A summary of the safety profile is as follows:
Dr. Guerrero-Ramos concluded the preliminary results of this clinical trial with the following concluding statements:
- The preliminary results suggest that chemohyperthermia in high risk non-muscle invasive bladder cancer patients seems at least not to be inferior to BCG in terms of efficacy
- Tolerability and safety profile are similar, with milder adverse effects and more irritative voiding problems associated with HIVEC
- These promising outcomes have to be confirmed after complete follow-up and in larger trials
Co-Authors: Daniel Antonio Gonzalez-Padilla, Alejandro Gonzalez-Diaz, Felipe Villacampa-Auba, Marta Rodriguez-Izquierdo, Carmen Gomez-Cañizo, Federico de la Rosa-Kehrmann, Alfredo Rodriguez-Antolin
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020
References:
- Fankhauser CD, Teoh JY, Mostafid H. Treatment options and results of adjuvant treatment in nonmuscle-invasive bladder cancer (NMIBC) during the Bacillus Calmette-Guerin shortage. Curr Opin Urol 2020 May;30(3):365-369.
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-Invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017 Mar;71(3):447-461.


