Between August 2017 and July 2019 this study was a multicenter prospective, blind study carried out at 21 centers. Patients over 18 years of age, with a previous diagnosis of non-muscle invasive bladder cancer undergoing cystoscopic surveillance, able to provide 10 mL of urine, and informed consent were recruited. Full void urine samples were collected and the ADXBLADDER test performed; ADXBLADDER results were compared to diagnosis obtained by cystoscopy and pathology. The study flow diagram is as follows:
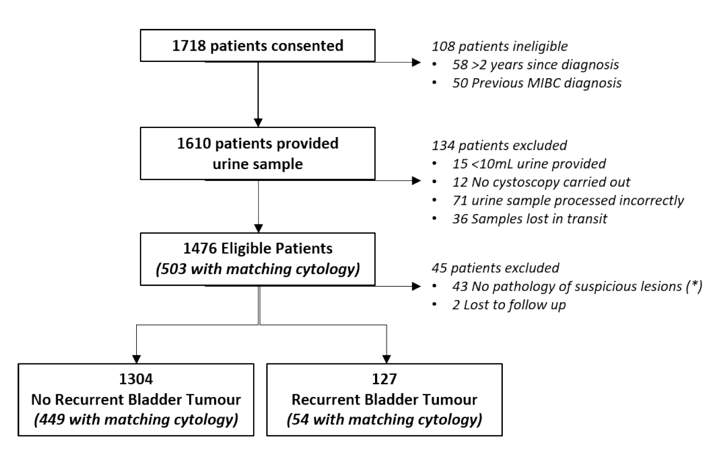
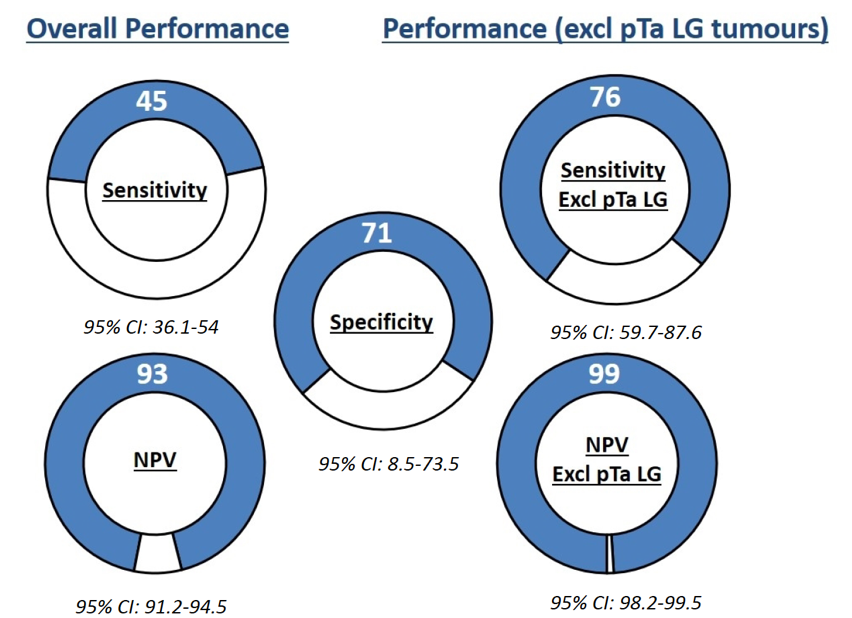
Of the 1,431 eligible patients enrolled, 127 bladder cancer recurrences were diagnosed (8.9% prevalence). Overall sensitivity for ADXBLADDER was 44.9% (95% CI 36.1%-54%) with specificity 71.1% (95% CI 68.5-73.5%) and NPV of 93% (95% CI 91.2-94.5%). ADXBLADDER sensitivity in detecting non-pTaLG tumors was 75.6% (95% CI 59.7-87.6%), with a NPV of 99.0% (95% CI 98.2-99.5%):
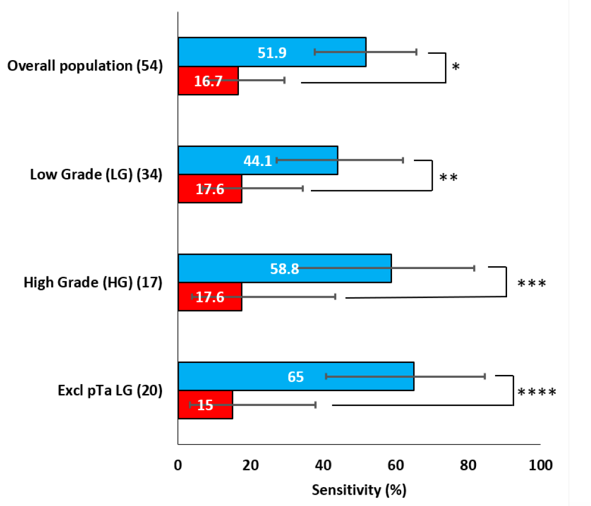
A subset of the study population (n=503) had matching cytology. In this sub-group ADXBLADDER demonstrated a significantly higher sensitivity of 51.9% (95% CI: 37.8-65.7%), versus the sensitivity of cytology 16.7% (95% CI 7.9-29.3%, p=0.0002):
There were no significant differences in the performance of ADXBLADDER due to age, sex, or treatment received.
Dr. Palou concluded his presentation of ADXBLADDER for detecting the recurrence of non-muscle invasive bladder cancer with the following take-home messages:
- This study demonstrates that in the follow up of non-muscle invasive bladder cancer patients, ADXBLADDER is able to exclude the presence of the most aggressive tumors with a NPV of 99%, with significantly higher sensitivity than cytology in all tumor types
- ADXBLADDER detected at least twice the number of recurrences than cytology for low-grade, high-grade, and CIS
- There were no significant differences in performance observed due to age, sex or treatment received
- These results reveal a compelling case for ADXBLADDER to replace cytology in the follow-up strategy of non-muscle invasive bladder cancer patients
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_m, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020


