For this study, participating institutions reviewed all patients treated with an induction course of sequential intravesical gemcitabine and docetaxel for recurrent non-muscle-invasive bladder cancer between June 2009 and May 2018. Inclusion criteria included those that had recurrent non-muscle-invasive bladder cancer and prior BCG therapy. Maintenance therapy was continued based on individual institutional protocols. Surveillance for recurrence was carried out per AUA guidelines. The primary endpoint was bladder recurrence-free survival and secondary endpoints were: high-grade recurrence-free survival, high-grade recurrence-free survival for BCG unresponsive patients, treatment tolerance, progression-free survival, cancer-specific survival, and overall survival. Recurrence and survival probabilities were plotted using the Kaplan-Meier methods and Cox regression models were used to evaluate the effect of patient, disease, and treatment variables on outcomes.
There were 276 patients included in the study with a median age of 73, with the most common BCG failure classification being unresponsive (38%); 60.9% of patients were deemed to be adequate candidates for cystectomy. There was a median follow-up of 22.9 months and a median number of two prior intravesical therapy induction courses among those included in the study. Only 3.3% of patients were unable to tolerate the full induction course of therapy. High-grade recurrence-free survival was 65% at 1 year and 52% at 2 years. Progression-free survival was 97% and 93% at 1 and 2 years, respectively. A summary of the 6-, 12-, and 24- month outcomes are as follows:
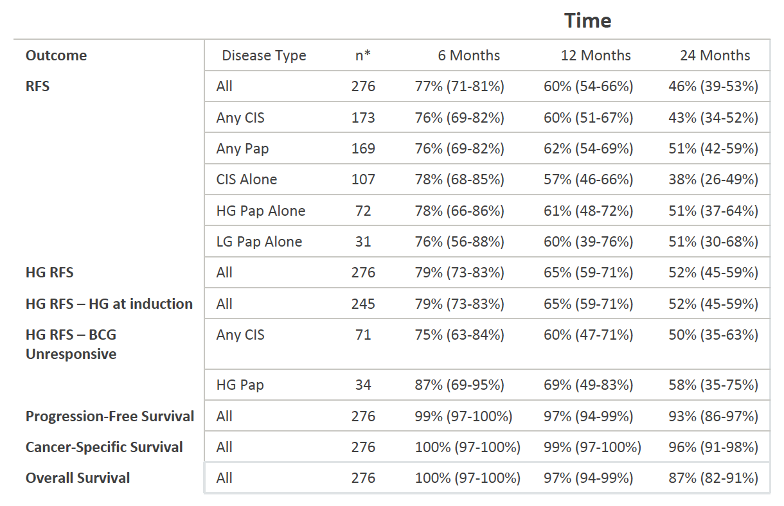
On Cox regression analysis, clinical-stage, number of prior BCG failure, BCG failure categorization, and treating institution did not impact disease recurrence. The addition of maintenance therapy was significantly associated with recurrence-free survival (HR 2.37, 95% CI 1.36-4.11, p<0.01). Sixteen percent of patients underwent cystectomy at a median of 11.3 months from starting therapy. Bladder cancer-specific mortality was 1% at 1 year and 5% at 2 years. There was no difference in outcome between patients with carcinoma in situ (CIS) and those with no CIS. In regards to treatment tolerance, 41% of patients experienced side effects, with 9.4% of patients with side effects having an impact on their treatment schedule. The most common side effects were urgency/frequency and dysuria.
Dr. Brooks concluded his presentation with the following take-home messages:
- In this multi-institutional review of a pretreated patient population with moderate follow-up, the intravesical administration of gemcitabine and docetaxel is safe, well-tolerated, and efficacious in preventing recurrence in patients with recurrent, non-muscle-invasive bladder cancer after BCG failure
- BCG failure number and category, clinical T stage, and the presence of CIS did not impact treatment success while the administration of maintenance therapy significantly improved RFS
- Gemcitabine and docetaxel are readily reconstituted, are generic, do not involve systemic infusion, and have not been plagued by supply shortages
- Prospective evaluation of gemcitabine and docetaxel, including possibly head to head evaluation with other emerging agents, is not only warranted but pragmatically necessary for patients with BCG failure and possibly BCG naïve non-muscle invasive bladder cancer
Presenter: Nathan Brooks, MD, MD Anderson Cancer Center, Houston, TX, USA
Co-Authors: Ryan Steinberg, Lewis Thomas, Sarah Mott, Andrew Vitale, Trafford Crump, Mounica Rao, Marcus Daniels, Jonathan Wang, Supriya Nagaraju, William DeWolf, Donald Lamm, Max Kates, Eric Hyndman, Ashish Kamat, Trinity Bivalacqua, Kenneth Nepple, Michael O'Donnell
Written By: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, GA, USA, Twitter: @zklaassen_md, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020
References:


