The goals of active surveillance are to:
- Avoid or delay the costs, both functionally and monetarily, of treatment without compromising cancer cure
- Compliant with screening and treatment guidelines.
- Based on the rationale that the initial assessment is reasonably accurate, and monitoring is accurate and identifies sub-clinical progression at a time that initial treatment options are still available and curative.
The impact of new technology, according to Dr. Carroll, is seen in the new genomic tests that are available. The 2019 National Comprehensive Cancer Network (NCCN) guidelines suggest that those with low-risk disease (T1-T2a, grade group 1, and PSA <10 ng/mL) should consider a genomic test if their life expectancy is >10 years, whereas those with the intermediate-risk disease (T2b-T2c, or grade group 2-3, or PSA 10-20 ng/mL) should consider a genomic test if the disease is favorable/intermediate-risk and if their life expectancy is >10 years. Clinically available genomic tests include Decipher, GPS, and Prolaris, all of which have prognostic value but not are predictive biomarkers.
The Oncotype DX GPS assay is a quantitative 17-gene RT-PCR assay on manually microdissected tumor tissue from needle biopsy. Genes and biological pathways are predictive of multiple endpoints, with an emphasis on clinical recurrence. This assay is optimized for very small tissue input with six 5 micron sections of a single needle biopsy block from as little as 1 mm of tumor length. Dr. Carroll’s group showed that GPS score was associated with upgrading on active surveillance in that a 5-unit increase in GPS score was associated with Gleason ≥3+4 disease (HR 1.27, 95% CI 1.18-1.38).2 Furthermore, a 5-unit increase in GPS score was associated with adverse pathology (HR 1.16, 95% CI 1.06-1.26) and PSA relapse (HR 1.10, 95% CI 1.00-1.21) among those undergoing delayed radical prostatectomy.3
Dr. Carroll notes that there is some concern with genomic classifiers, including:
- The issue with tumor heterogeneity
- Do all patients benefit? It is unlikely that very low risk patients benefit from a genomic assay, but which low and favorable intermediate risk patients do?
- Are they cost-effective?
- What are the long-term outcomes?
There are several current controversies in active surveillance according to Dr. Carroll:
- What risk categories are appropriate? Very low risk, low risk, those with low CAPRA scores, those with Gleason 3+4 disease?
- Is it safe in African American men?
- Are younger men appropriate candidates?

On Cox regression analysis, younger age was independently associated with lower risk of biopsy-based Gleason score upgrade (HR per 1-year decrease: 0.969, 95% CI 0.956-0.983]), and persisted upon restriction to men meeting strict active surveillance inclusion criteria. Furthermore, there was no significant association between younger age and risk of definitive treatment or risk of biochemical recurrence after delayed radical prostatectomy.
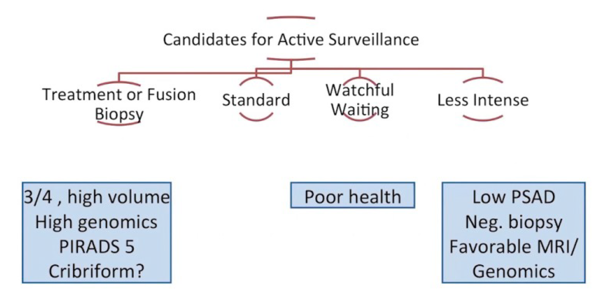
Whether all men with Gleason 3+4 disease should undergo an immediate radical prostatectomy is unclear. Dr. Carroll notes that cancer risk is best assessed with multivariable instruments and not as a single variable. Furthermore, radical prostatectomy only has an (arguably marginal) benefit in those with palpable tumors, higher-risk disease, or those with a PSA > 10 ng/mL. Additionally, Gleason 3+4 disease alone may only add a little risk (1 point in CAPRA), and volume (rather than grade alone) is a predictor of adverse pathology. It is also important to note that the subtype of Gleason pattern 4 disease is important (ie. expansile, poorly formed, or fused). Those with cribriform histology and stromal reaction are associated with higher genomic scores and the risk of extracapsular extension/recurrence compared to glomerulation and no stromal reaction.
Dr. Carroll highlighted the following cautionary remarks for men with Gleason 3+4 considering active surveillance:
- High PSA density
- PI-RADS 5 on mpMRI
- Adverse genomics (ie. GPS score)
- Cribriform (expansile) histology
- Larger number of biopsy cores positive at diagnosis (volume)
To conclude his Ramon Guiteras lecture on active surveillance for prostate cancer, Dr. Carroll provided the following take-home messages:
- Active surveillance is the preferred form of treatment for men with very low, low-risk and selected patients with favorable intermediate-risk disease
- New technology (MRI and genomics) appears to make it safer
- Its use remains highly variable, particularly in the United States
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, August, GA, USA, Twitter: @zklaassen_md, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020
References:
- Balakrishnan AS, Cowan JE, Cooperberg MR, et al. Evaluating the safety of active surveillance: Outcomes of deferred radical prostatectomy after an initial period of surveillance. J Urol 2019 Sep;202(3):506-510.
- Kornberg Z, Cowan JE, Westphalen AC, et al. Genomic Prostate Score, PI-RADS Version 2 and Progression in Men with Prostate Cancer on Active Surveillance. J Urol 2019 Feb;201(2):300-307.
- Kornberg Z, Cooperberg MR, Cowan JE, et al. A 17-Gene Genomic Prostate Score as a Predictor of Adverse Pathology in Men on Active Surveillance. J Urol 2019 Oct;202(4):702-709.
- Welty CJ, Cowan JE, Nguyen H, et al. Extended Followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol 2015 Mar;193(3):807-811.
- Leapman MS, Cowan JE, Nguyen HG, et al. Active surveillance in Younger Men with Prostate Cancer. J Clin Oncol 2017 Jun 10;35(17):1898-1904.


