Prostate cancer is the most common cancer diagnosis and the second most common cause of cancer death in US men, as shown in Figure 1. It is also known that cardiovascular mortality is the leading cause of death in patients with prostate cancer (Figure 1).
Figure 1: Incidence and burden of advanced prostate cancer:

Androgen deprivation therapy can be given in the form of an LHRH receptor agonist or GnRH receptor antagonist (Figure 2), with both causing testosterone castration levels.
Figure 2: Androgen deprivation therapy – mechanism of testosterone suppression:

Dr. Shore moved on to present the HERO trial, A Study to Evaluate the Safety and Efficacy of Relugolix in Men With Advanced Prostate Cancer. This was a phase III multinational, randomized, open-label, parallel-group study to evaluate the safety and efficacy of relugolix in men with advanced prostate cancer. The primary endpoint was sustained castration through 48 weeks with testosterone levels less than 50 ng/dl. Figure 3 shows the study design and Table 1 demonstrates the key eligibility criteria. Figure 4 depicts the patient disposition in the HERO study.
Figure 3: HERO study design:
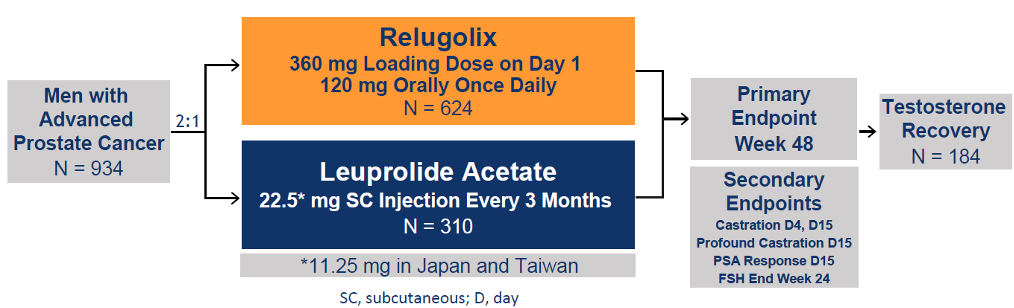
Table 1: Key eligibility criteria:


Figure 4: HERO study:
Continuing his presentation, Dr. Shore presented the demographic data of the patients enrolled in the HERO trial (Table 2), and their clinical characteristics (Table 3).
Table 2: Demographic data of patients in the HERO trial:

Table 3: Clinical characteristics of patients in the HERO trial:

When assessing the primary endpoint, sustained castration was noted in 96.7% of patients on relugolix and 88.8% of patients on leuprolide, with a between-group difference of 7.9% (95% confidence interval [CI] 4.1-11.8%), as seen in Figure 4.
Figure 4: Sustained castration:

The secondary endpoints were presented next, showing again, a higher proportion of patients with prostate-specific antigen (PSA) response at day 15, a higher probability of testosterone suppression, and a lower mean follicle-stimulating hormone (FSH) level (Table 4).
Table 4: Key secondary endpoints:

Figure 5 depicts the time course of testosterone suppression throughout the study period. Additionally, the testosterone recovery was faster with relugolix compared to leuprolide (Figure 6).
Figure 5: Testosterone suppression:

Figure 6: Testosterone recovery:

The rate of adverse events was quite similar to any adverse event occurring n 92.9% and 93.5% in the relugolix and leuprolide groups, respectively. Fatal adverse events occurred in 1.1%, and 2.9% of patients treated with relugolix and leuprolide, respectively. The most common adverse events are shown in Table 5.
Table 5: Common adverse events:

Interestingly, cardiovascular adverse events occurred in 7.1% of leuprolide patients compared to 3.9% of relugolix patients. The results demonstrated an impressive 54% reduction in the risk of major adverse cardiovascular events (MACE), as shown in Figure 7.
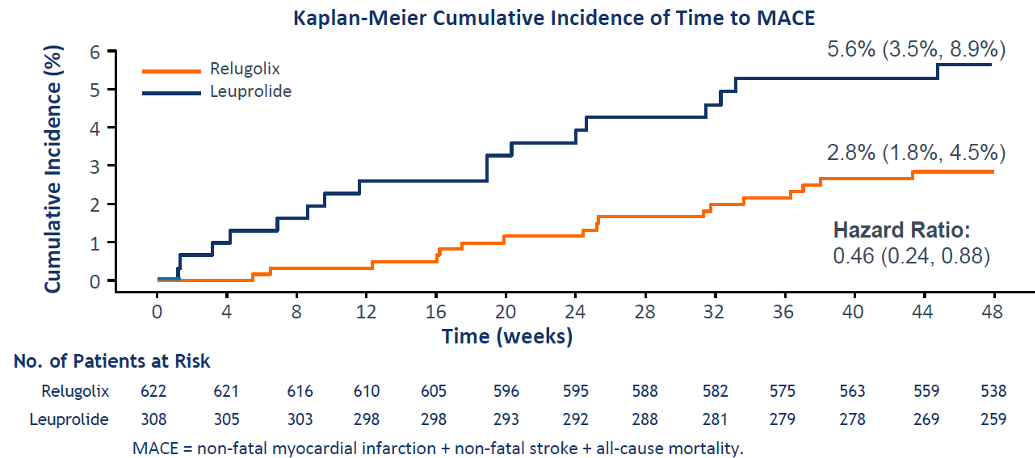
Figure 7: Major adverse cardiovascular events:
Dr. Shore concluded his talk stating that all of the primary and key secondary endpoints were achieved in the HERO trial. Ninety-six point seven percent achieved and sustained castration through week 48. Relugolix achieved superiority over leuprolide in sustained castration rates, castration and profound castration by day 15, and PSA response by day 15. Relugolix was also superior in achieving better testosterone recovery rates within the normal range (54% vs. 3%) at day 90.
Lastly, relugolix once-daily oral therapy was well-tolerated, and the risk of major adverse cardiovascular events was 54% lower than with leuprolide.
Presented by: Neal Shore, MD, FACS, Medical Director of the Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC, USA
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, New York, USA, Twitter: @GoldbergHanan, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020.
Related Content:
Watch: HERO Phase III Trial: Results Comparing Relugolix, an Oral GnRH Receptor Antagonist Vs. Leuprolide Acetate for Advanced Prostate Cancer - Neal Shore
Read: ASCO 2020: HERO Phase III Trial: Results Comparing Relugolix, an Oral GnRH Receptor Antagonist, versus Leuprolide Acetate for Advanced Prostate Cancer


