(UroToday.com) Neoadjuvant systemic therapy is a widely established treatment paradigm for many malignancies. However, the use of androgen deprivation therapy is not widely endorsed in patients with non-castrate-resistant prostate cancer. The use of neoadjuvant hormonal therapy has been shown previously to improve pathological outcomes reducing positive surgical margins and increase the incidence of organ-confined disease. However, no survival benefit was ever shown with the use of neoadjuvant hormonal therapy.
It is known that men with high-risk prostate cancer have a 50% risk of enduring biochemical recurrence with surgical treatment alone within 10 to 15 years. Neoadjuvant hormonal therapy can reduce the tumor burden, enabling the performance of a nerve-sparing approach in men with high-risk disease, and increasing their quality of life after surgery.
The newer generation of anti-androgens is more effective at causing castration. This might improve oncological outcomes that were not seen in earlier studies using older generation anti-androgens.There has been no head to head comparison of neoadjuvant treatment, using the latest generation of anti-androgen medications, followed by radical prostatectomy to immediate radical prostatectomy.
The currently presented study was a randomized phase 3 prospective three-arm trial with a planned postoperative follow up of two years. The primary objective was to evaluate the effect of neoadjuvant androgen deprivation treatments on the feasibility of performing a nerve-sparing radical prostatectomy in patients with high-risk prostate cancer (study design is shown in Figure 1).
Figure 1 – Study design: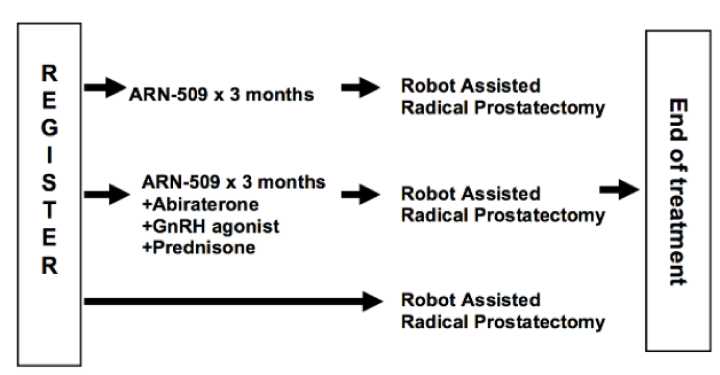
The primary outcome was post-surgical potency rate at 12 months, defined as being able to penetrate and complete sexual intercourse satisfactorily in more than 50% of the attempts. Each of the experimental arms of this trial would be compared to the surgery the only arm.
Secondary outcomes included change in tumor volume on pelvic MRI after neoadjuvant therapy which were correlated with clinical outcomes before and after treatment with the androgen receptor antagonist (ARN-509, apalutamide for 3 months) or the combination of androgen receptor antagonist (ARN-509, apalutamide for 3 months) + GNRH agonist + prednisone + abiraterone. Other secondary outcomes included biochemical recurrence rate, pathological T0, positive surgical margins rate, postoperative continence rate (being pad free), and quality of life assessed by the EPIC questionnaire.
A total of 32 patients were enrolled since November 2019, with 24 patients remaining in the follow-up phase. There was no significant difference in the operative time between the three arms. The median reduction of tumor volume was 32.4% and 55.7% in arm 1 and arm 2, respectively. Positive surgical margins were noted in 3,1 and 3 patients in arms 1,2, and 3, respectively. Potency was achieved by 50%, 14.2%, and 28.5% of the patients in arms 1,2, and 3. Biochemical recurrence occurred in 1,2, and 0 patients in arms 1,2, and 3, respectively. Table 1 demonstrates the operative outcomes in all 3 arms.
Table 1 – Operative outcomes: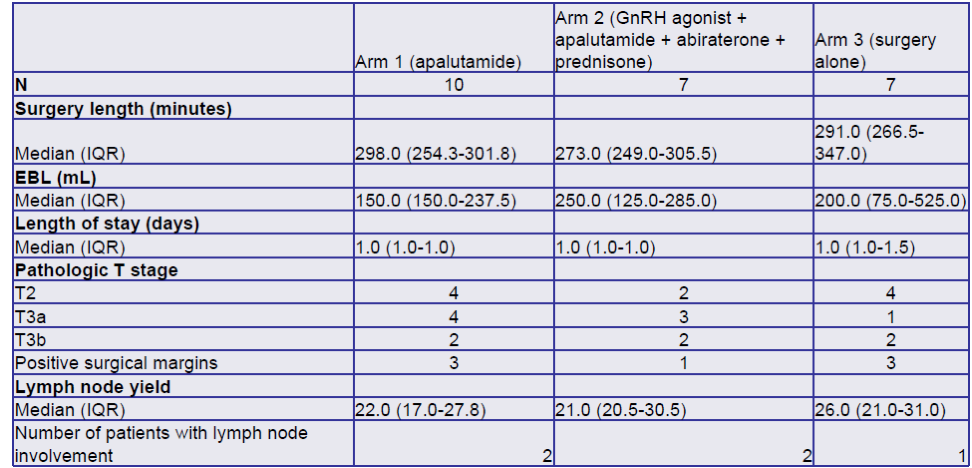
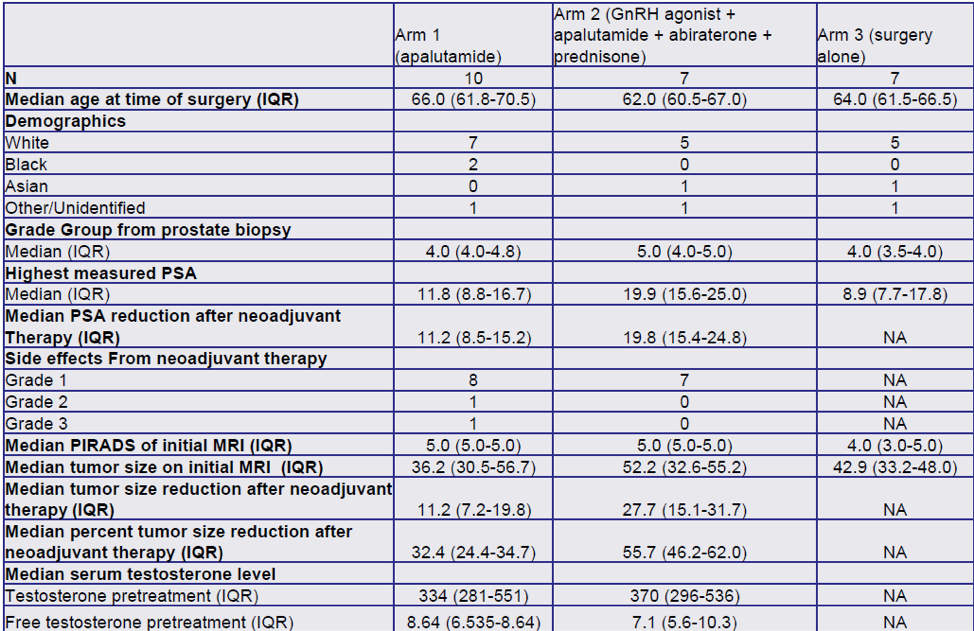
The authors concluded that these early results might indicate that neoadjuvant therapy before radical prostatectomy can result in a reduction of tumor volume. These early data suggest improved potency preservation without an adverse effect on positive surgical margin rates. However, more data is needed to validate these initial results.
Presented by: Joshua Sterling, MD, Robert Wood Johnson Medical School, New York, NY, USA
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020.


