It is important to note that the treatment benefit of enzalutamide plus ADT on the primary endpoint of radiographic progression-free survival in the ARCHES trial was observed regardless of baseline PSA level at study entry.
Improvements in other PSA related outcomes in the overall population were also observed. The inclusion of patients regardless of their disease volume and prior chemotherapy in the ARCHES trial enables additional insights on treatment efficacy in this specific patient population.
The objective of this presented study was to assess the PSA related outcomes in the ARCHES trial, stratified by disease volume (low vs. high) and prior docetaxel therapy (with vs. without) at study entry.
The ARCHES study design is depicted in Figure 1, and baseline patient characteristics are shown in Table 1.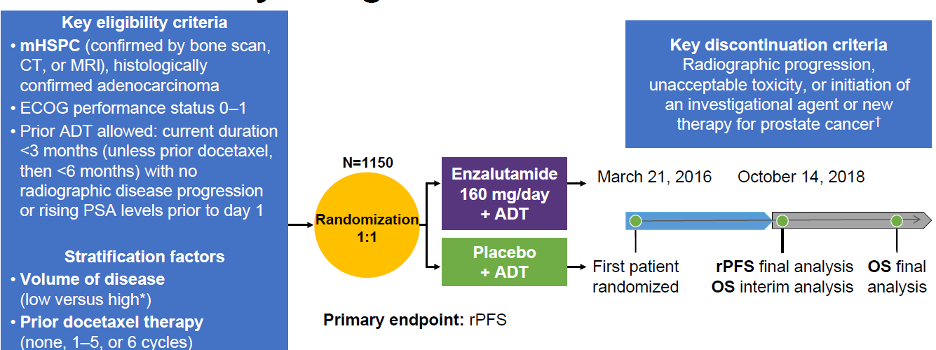
Table 1 - Baseline patient characteristics:
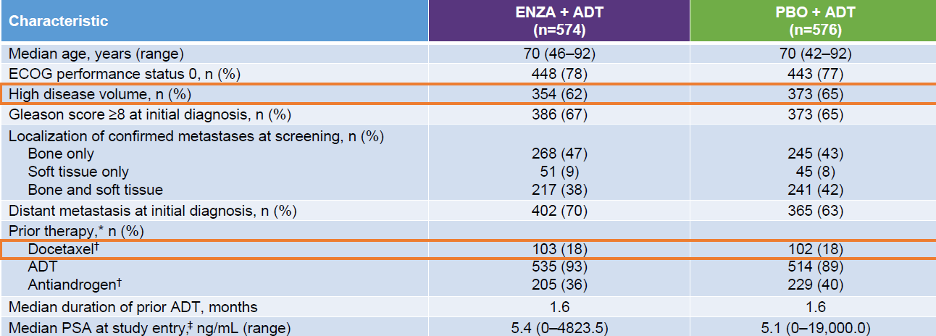
Figure 2 demonstrates the time to PSA progression stratified by disease volume. This shows a reduced risk of PSA progression with enzalutamide and ADT vs. placebo and ADT in patients with both low (92%) and high volume (78%) disease. Figure 3 demonstrates time to PSA progression stratified by prior treatment with docetaxel. This also shows a reduced risk of PSA progression with enzalutamide and ADT vs. placebo and ADT in patients with no prior docetaxel chemotherapy (82%) or 1-6 cycles of prior docetaxel chemotherapy (78%). Lastly, Figure 4 demonstrates the time to 50% PSA reduction in the overall population.
Figure 2 – Time to PSA progression stratified by disease volume: 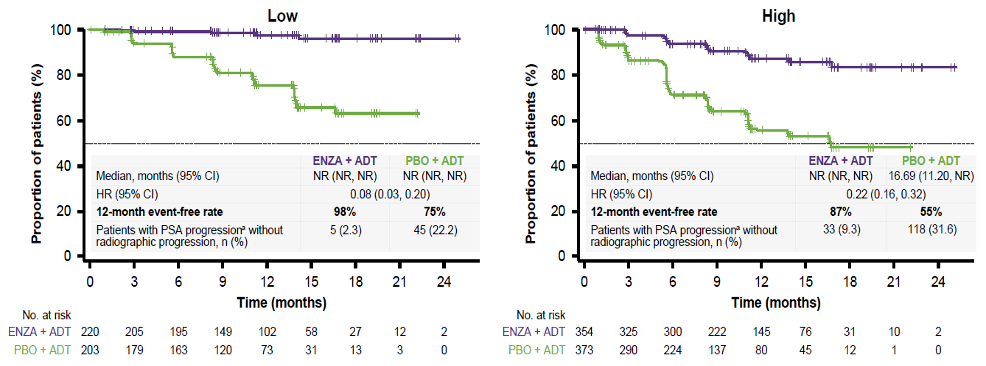
Figure 3 – Time to PSA progression stratified by prior docetaxel chemotherapy: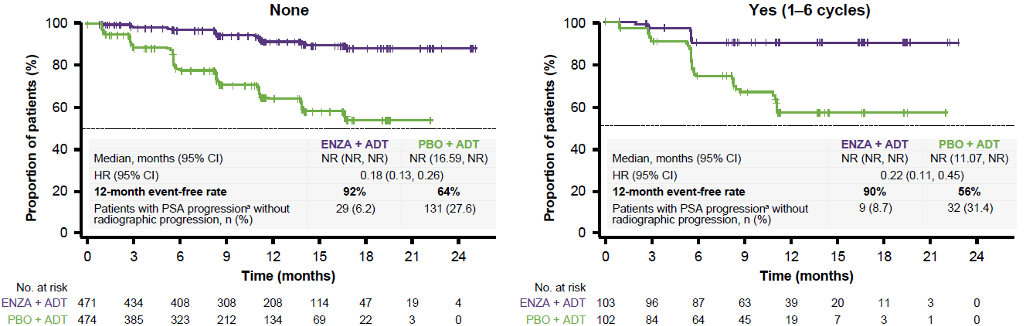
Figure 4 – Time to 50% PSA reduction in the overall population:
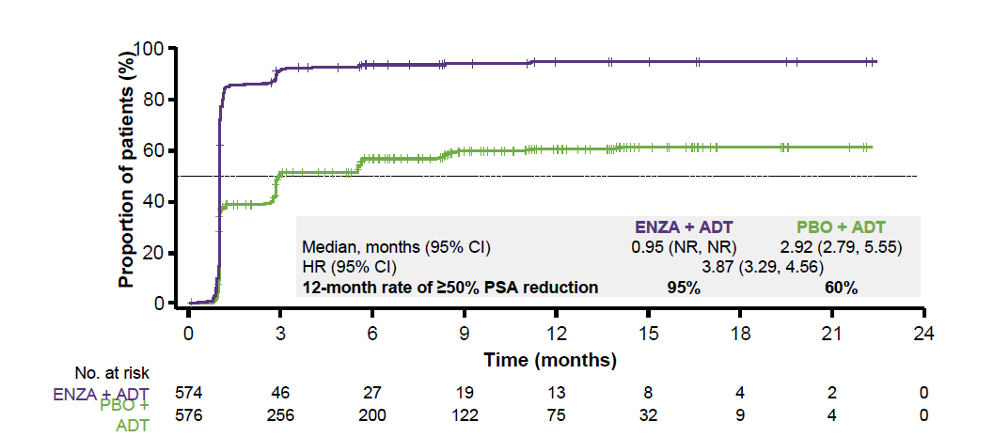
Consistent with the overall population, the median time to 50% PSA reduction was shorter with treatment with enzalutamide and ADT regardless of disease volume or prior docetaxel. A greater proportion of patients receiving enzalutamide + ADT achieved >=50% PSA reduction from baseline, regardless of disease volume or prior docetaxel chemotherapy (Figure 5).
Figure 5 – Time to 50% PSA reduction: 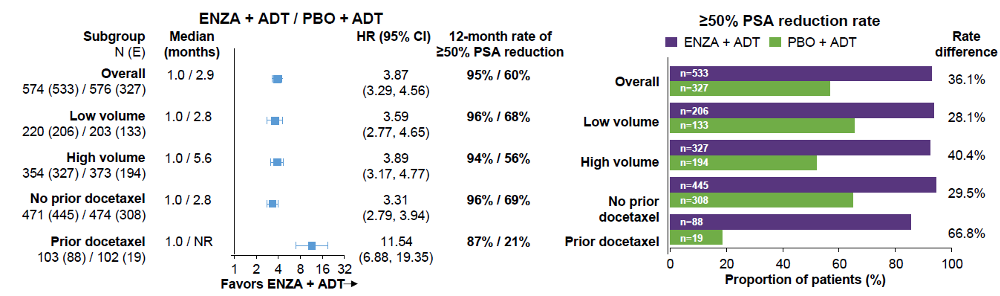
In patients with detectable PSA (>=0.2 ng/ml) at baseline, the median time to undetectable PSA was shorter with enzalutamide and ADT (5.5 months) vs. placebo + ADT (not reached) in the overall population. The median time to undetectable PSA was shorter with enzalutamide + ADT regardless of disease volume or prior docetaxel.
In conclusion, when comparing enzalutamide plus ADT to placebo plus ADT in this post-hoc analysis of patients with metastatic hormone-sensitive prostate cancer, enzalutamide plus ADT resulted in:
- A long term reduced risk of PSA progression
- A more rapid and long-term reduction in PSA from baseline
Importantly, the demonstrated treatment benefit of enzalutamide plus ADT on PSA related outcomes was observed regardless of disease volume or prior docetaxel therapy at study entry.
Presented by: Neal Shore, MD, FACS, Carolina Urologic Research Center, Myrtle Beach, SC
Written By: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020
References:
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. Journal of Clinical Oncology. 2019;37(32):2974-86.
- Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. New England Journal of Medicine. 2019;381(2):121-31.


