Figure 1 -CARD study design:

The results demonstrated a significant advantage in patients treated with cabazitaxel in both overall survival and radiographic progression-free survival (Figure 2).
Figure 2 – Radiographic progression-free survival and overall survival:

There was also a significant advantage for cabazitaxel in pain response and pain progression, as seen in Figure 3. Furthermore, there was a clear advantage to cabazitaxel with less symptomatic skeletal events (Figure 4).
Figure 3 – Pain response and pain progression:

Figure 4 – Symptomatic skeletal events:

Cabazitaxel was shown to have a higher rate of febrile neutropenia, but a lower rate of cardiac disorders compared to abiraterone or enzalutamide but generally had a manageable safety profile.
In the currently presented study, the authors planned to prospectively evaluate changes in the quality of life in patients receiving cabazitaxel compared to those receiving abiraterone or enzalutamide, using the EQ-5D-5L utility index and visual analog scale (VAS) (Figure 5).
Figure 5 –

The baseline patient characteristics are shown in Table 1. The mean VAS at baseline was 65.8 with cabazitaxel (n=113) and 66.3 with abiraterone or enzalutamide (n=112). The mean utility score at baseline was 0.7 for both treatment arms (Figure 6). Similar trends were seen across all EQ-5D-5L domains, as seen in Figure 7.
Table 1 – Baseline patient characteristics:

Figure 6 – Changes in VAS and utility index:

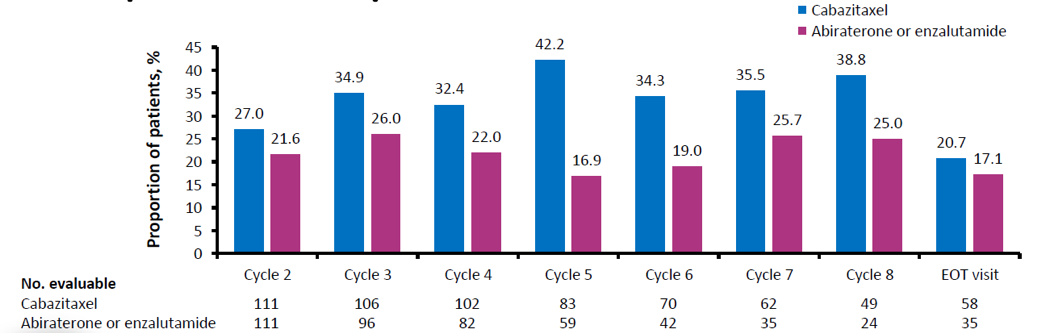
Figure 7 – EQ-5D-5L pain/discomfort proportion improved from baseline:
The authors concluded that the CARD study met its primary objective with cabazitaxel more than doubling the radiographic progression-free survival compared to abiraterone or enzalutamide. Furthermore, cabazitaxel reduced the risk of death by 36% and improved pain, time to pain progression, and time to symptomatic skeletal events.
The safety profile of cabazitaxel was manageable, with 3% of patients developing febrile neutropenia. Lastly, the changes in VAS and the utility score were better with cabazitaxel.
These results further support the use of cabazitaxel over abiraterone or enzalutamide as a standard of care in patients previously treated with docetaxel, who progressed within 12 months with the alternative therapy of abiraterone or enzalutamide.
Presented by: Gero Kramer, MD, Department of Urology, Medical University of Vienna, Vienna, Austria
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020.
References:


