(UroToday.com) Patients who were treated with enzalutamide have shown increased expression of PD-L1 in circulating dendritic cells. Patients with greater PD-L1 expression were found to have worse response rates to enzalutamide treatment.1
There is a phase 2 single-institution study that assessed pembrolizumab + enzalutamide in patients whose disease progressed after enzalutamide (n=28)(2). In these patients, the radiographic response rate was 25%. Furthermore, 18% had a greater than 50% decline in PSA from baseline and a median overall survival of 22.2 months(2).
Initial data from the cohort C of KEYNOTE-365 showed that pembrolizumab + enzalutamide are generally well-tolerated and showed promising activity in patients with metastatic castrate-resistant prostate cancer, previously treated with abiraterone.
In this presentation, the authors present updated data from the KEYNOTE-365 cohort C after additional enrollment, extended follow-up, and with more radiologic data.
The study design of KEYNOTE 365 cohort C is shown in Figure 1, the patient disposition is shown in figure 2, and the baseline characteristics are shown in Figure 3.
Figure 1 – KEYNOTE 365 - COHORT C: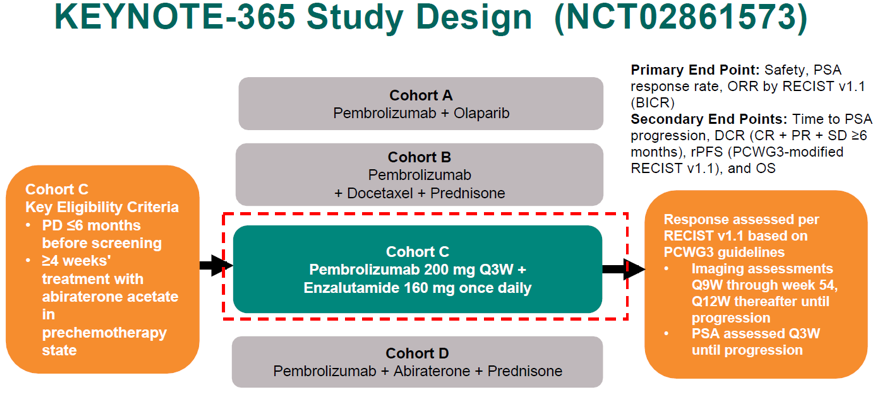
Figure 2 – Patient disposition: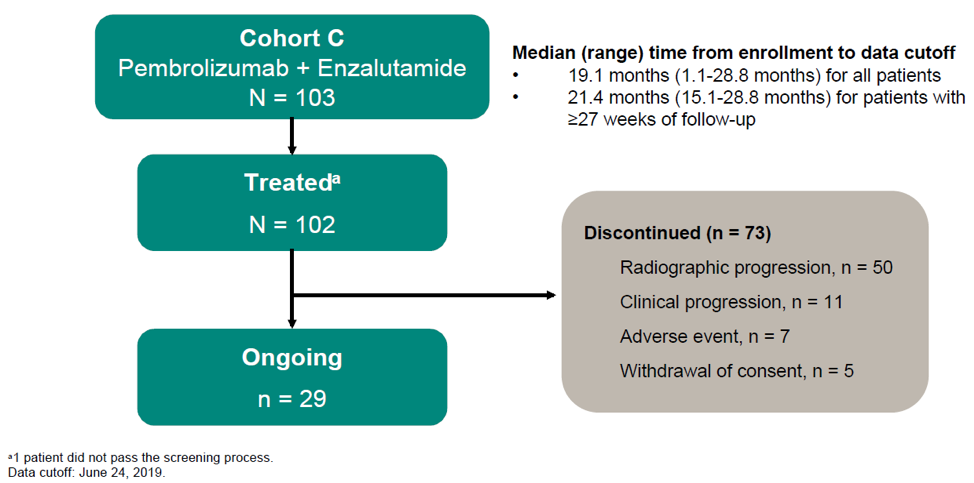
Figure 3 – Baseline characteristics: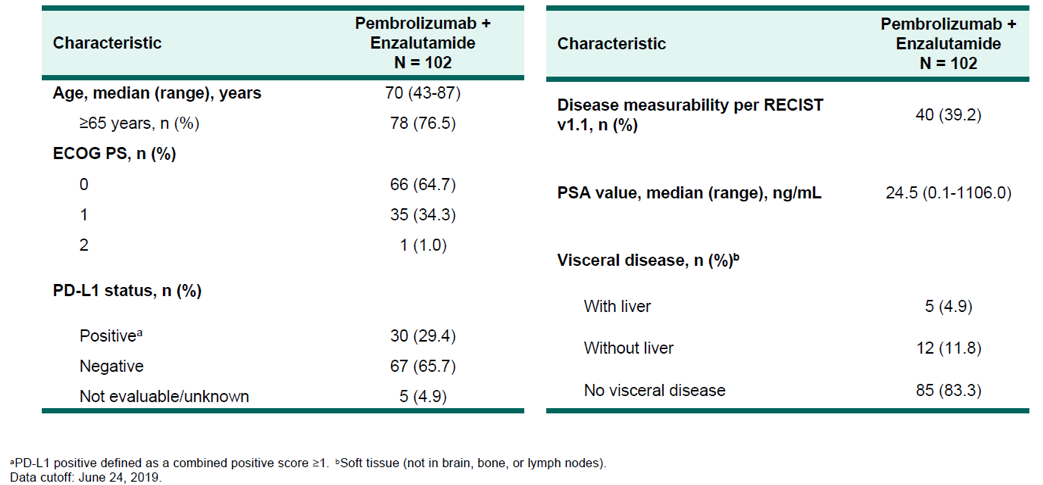
Figure 4 demonstrates the confirmed PSA response rate and the percentage change from baseline. Figure 5 depicts the time to PSA progression. A total of 56% of patients experienced a reduction in target lesions, and 24% experienced a reduction greater than 30%. Figure 6 shows the radiographic progression free-survival and overall survival.
Figure 4 – PSA response rate and percent change from baseline: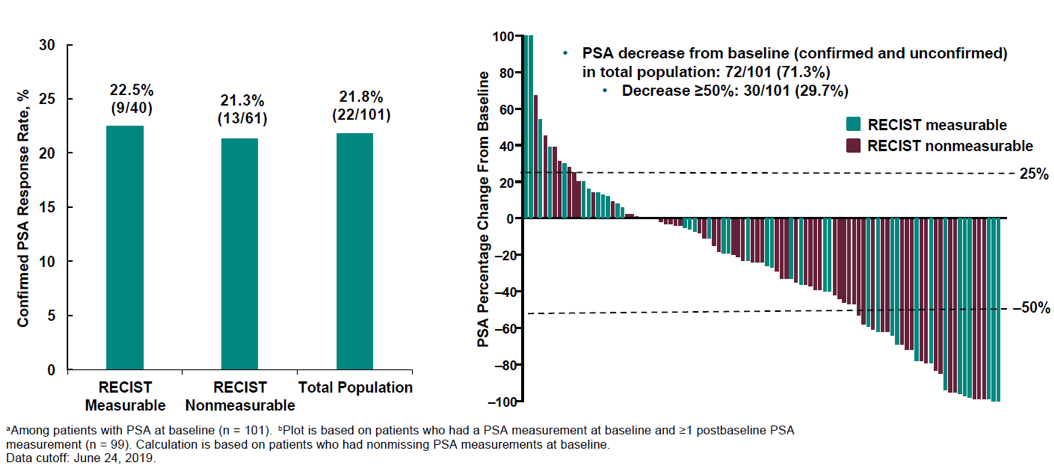
Figure 5 – Time to PSA progression: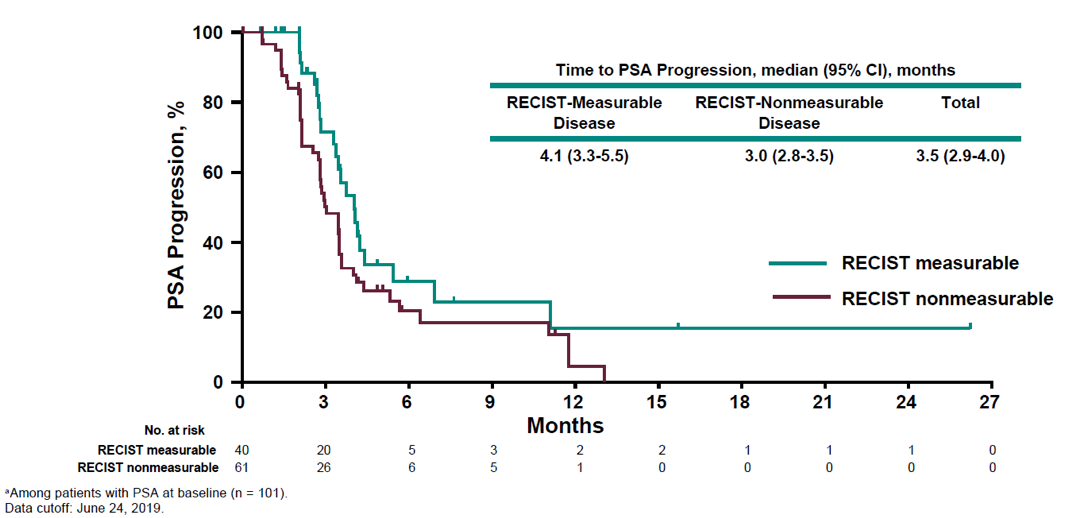
Figure 6 – Radiographic progression-free survival and overall survival: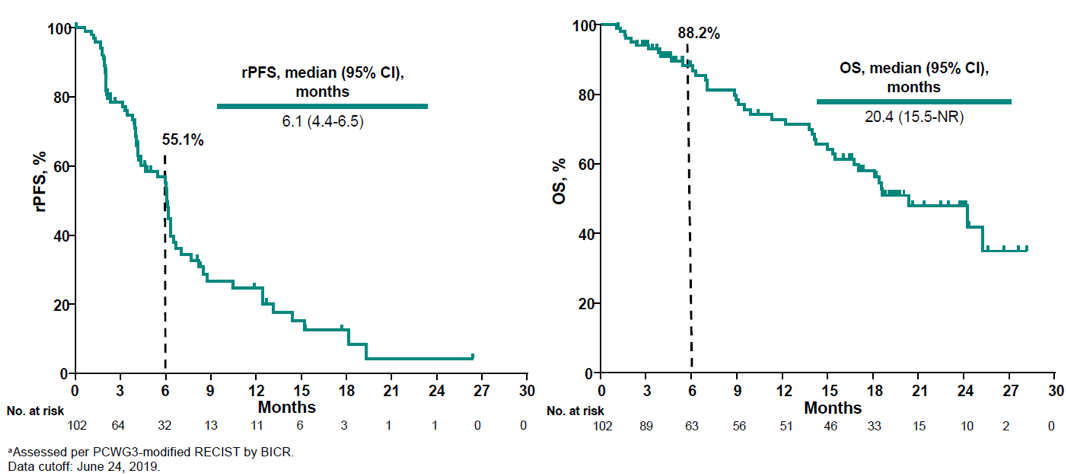
The treatment-related adverse effects showed that 90.2% of patients experienced any kind of adverse effect, while 21.6% experienced grade 3-5 adverse effects.
The authors concluded that this data that had incorporated increased enrollment and additional follow up showed that pembrolizumab and enzalutamide continue to show promising activity in patients with metastatic castrate-resistant prostate cancer, who were previously treated with abiraterone. This promising activity manifested in:
- Confirm the PSA response rate for the total population of 21.8%
- objective response rate per RECIST v1.1 in patients with measurable disease of 12%
- Disease Control rate with a total population of 34.8%
- radiographic progression-free survival of 6.1 months with overall survival of 20.4 months
- The safety and tolerability profile of pembrolizumab and enzalutamide was consistent with that of previously published data. There was an increased incidence of all grade rash (19.6%) and grade 3 rash (7.8%), which resolved with the standard of care treatment.
The promising results of this study support further evaluation of pembrolizumab and enzalutamide in patients with metastatic castrate-resistant prostate cancer who were previously treated with abiraterone.
There is currently an ongoing randomized phase 3 study of enzalutamide with or without pembrolizumab, that is open to enrollment (KEYNOTE-641, NCT03834493) for patients with metastatic cancer-resistant prostate cancer who did not receive chemotherapy, and who are intolerant to or progressed on or after abiraterone acetate therapy.
Presented by: Evan Yu, MD, Professor, University of Washington, Seattle, WA, USA
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020.
References:
- Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2015;6(1):234-42.
- Graff JN, Alumkal JJ, Thompson RF, Moran A, Thomas GV, Wood MA, et al. Pembrolizumab (Pembro) plus enzalutamide (Enz) in metastatic castration resistant prostate cancer (mCRPC): Extended follow up. Journal of Clinical Oncology. 2018;36(15_suppl):5047-.
Read: Keynote-365 cohort a: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC).
Read: ASCO GU 2020: KEYNOTE-365 Cohort A Updated Results: Pembrolizumab Plus Olaparib in Docetaxel-Pretreated Patients with mCRPC


