The Be-Well Study, a prospective cohort of NMIBC patients treated at Kaiser Permanente Northern California from 2015-2019, was queried for patients meeting criteria for intermediate-risk NMIBC (any one of recurrent low-grade Ta within one year, or initially low-grade Ta either >3 cm or multifocal, low-grade T1 or high-grade Ta < 3 cm). Exclusion criteria were (i) AUA low-risk NMIBC, (ii) AUA high-risk NMIBC, (iii) initial muscle-invasive or metastatic bladder cancer, (iv) age < 21 years, (v) initial non-urothelial histology, and (vi) histologic variants. Adverse events were identified using a combination of electronic medical record review, ICD-10-CM codes, CPT codes, and pharmacy prescription data. The primary outcome was any adverse event within 90 days of TURBT. Adverse events of interest included UTI (non-prophylactic prescription of a urinary antibiotics for symptoms or a positive urine culture), lower urinary tract symptoms (requiring a new non-prophylactic anticholinergic prescription), and urinary retention (new onset requiring repeat catheterization). Statistical analysis included logistic regression for adverse events and Kaplan-Meier analysis for association of adverse events with recurrence.
In a prospective cohort of 291 intermediate-risk NMIBC patients the baseline patient characteristics are as follows:

Overall, the initial TURBT adverse event rate was 32.7% with the most common being: urinary tract infection (15.1%), lower urinary tract symptoms requiring a new anticholinergic prescription at least 8 days after TURBT (10.7%), and urinary retention requiring catheter replacement (7.6%). The Clavien grade III/IV incidence of complications was 2.4%:

Among patients that had a re-TURBT, the 90-day adverse event rate was 33% and no different from the initial TURBT data. Patients who had at least one adverse event had a 61% probability of a second adverse event; 19.9% of patients had 2+ adverse events. Notably, 71.4% (15/21) of patients who received an anticholinergic within 90 days of TURBT re-filled their prescription at least once after 90 days beyond TURBT. ICD-10-CM codes alone had a sensitivity of 58% for detecting adverse events, but missed urinary tract infections, anticholinergic prescriptions, and codes for re-catheterization. Multivariable logistic regression identified females, higher Charlson Comorbidity Index, and high-grade tumors as predictors of adverse events:

Adverse events were also significantly associated with recurrent disease:
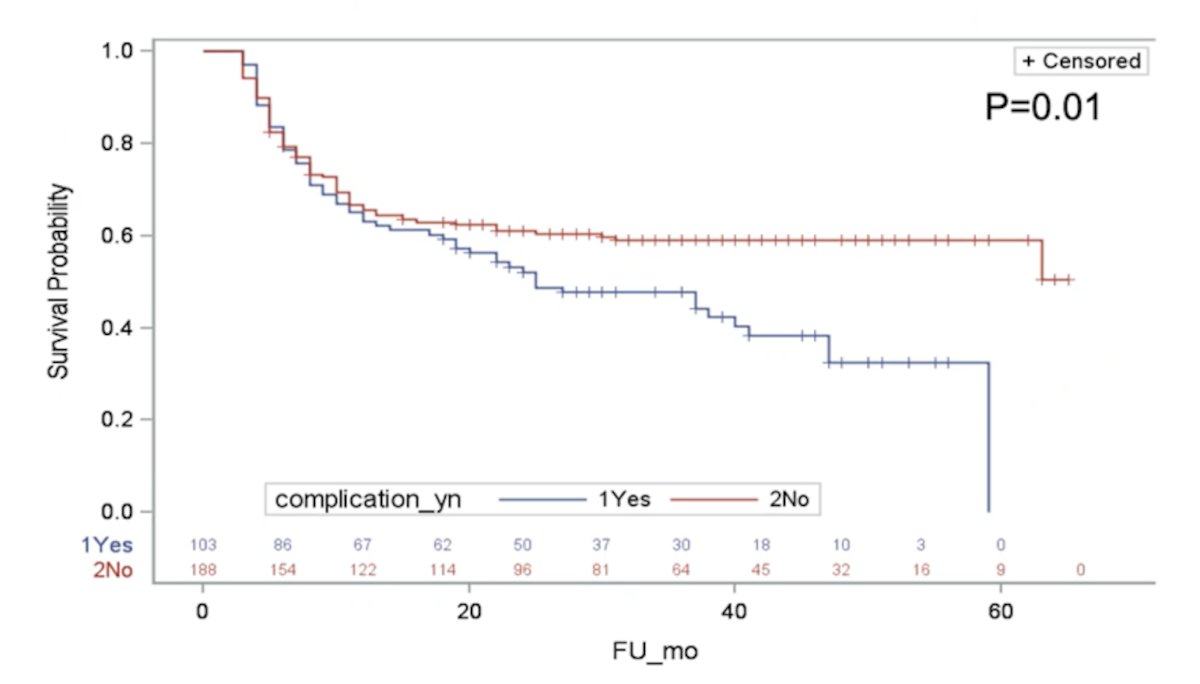
Dr. Sharma concluded his presentation with the following summary points:
- About one-third of patients undergoing TURBT will experience an adverse event within 90 days, and about one-third of them will experience a second adverse event
- These results suggest that the existing literature may underestimate the impact of TURBT
- The most common adverse events unreliably captured by codes were UTI, lower urinary tract symptoms requiring an anticholinergic, urinary retention, and sepsis
- These data can provide more accurate expectations to assist patient counseling prior to TURBT and identify opportunities to reduce adverse events
Presented by: Vidit Sharma, MD, Department of Urology, University of California-Los Angeles, Los Angeles, CA
Co-Authors: David S Aaronson, Katherine E Fero, Patrick M Lec, Karim Chamie, Valerie S Lee, Charles Quesenberry, Julie R Munneke, Mark Schoenberg, Lawrence H Kushi, Li Tang, Marilyn L Kwan
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.
References:
- Matulewicz RS, Sharma V, McGuire BB, et al. The effect of surgical duration of transurethral resection of bladder tumors on postoperative complications: An analysis of ACS NSQIP data. Urol Oncol. 2015 Aug;33(8):338.e19-24.


