(UroToday.com) Most patients newly diagnosed with bladder cancer have non-muscle invasive disease (NMIBC). For patients with intermediate or high-risk NMIBC and those with carcinoma in situ (CIS), adjuvant treatment with BCG is guideline-recommended on the basis of proven benefits in disease recurrence. In contrast, for those with low-grade intermediate-risk non-muscle-invasive bladder cancer (LG IR NMIBC), the standard of care is transurethral resection under general anesthesia. However, LG IR NMIBC is a highly recurrent malignancy, and patients often endure repeated surgeries that may be associated with significant postoperative and long-term morbidity.
In a podium presentation at the American Urologic Association Annual Meeting, Dr. William Huang and colleagues presented an analysis of the efficacy and safety of UGN-102, a mitomycin-containing reverse thermal gel, as primary chemoablative therapy in patients with LG IR NMIBC.

The authors performed a prospective, Phase 2b, open-label, single-arm trial of adult patients with biopsy-proven LG IR NMIBC. Patients received treatment with 6 once-weekly instillations of UGN-102 in an office setting. The primary endpoint was complete response (CR), defined as negative endoscopic examination, negative cytology, and negative for-cause biopsy at 3 months after treatment initiation. Patients who achieved CR were followed quarterly up to 12 months to assess durability of response and this was estimated by the Kaplan-Meier method. Safety was monitored throughout.
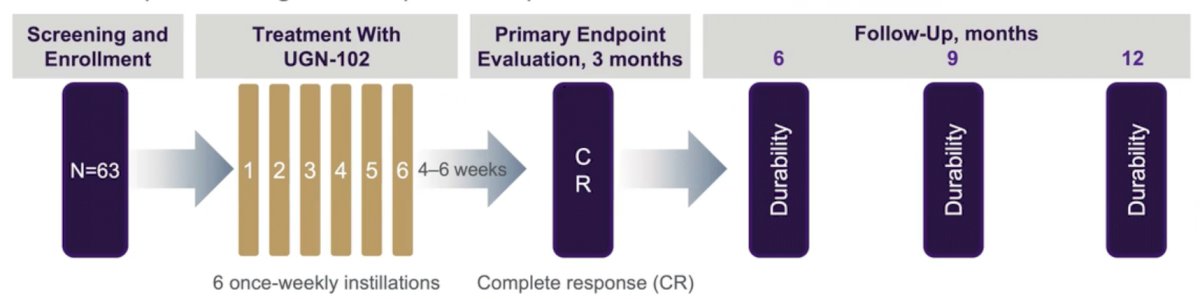
The authors enrolled a total of 63 patients (mean age 71 years [range 33-96], 60.3% male) who were treated with UGN-102 and formed the intent-to-treat population. 78% of patients had recurrent LG NMIBC, and 44% had a previous episode within 1 year of the current diagnosis. Recurrent patients had a mean (range) of 4.4 (1-12) prior NMIBC episodes and underwent a mean (maximum) of 4.0 (13) prior TURBTs.
Among the 63 patients, 41 (65%) patients achieved CR at 3 months, of whom 39 (95%), 30 (73%), and 25 (61%) remained disease-free at 6, 9, and 12 months after treatment initiation, respectively. Among the 43 patients who had a CR at 3 months, 13 (32%) had subsequent disease recurrence. Thus, the 9-month DOR rate (the probability that a patient will maintain CR for at least 9 months) was 73%.

In terms of toxicity, adverse events were primarily mild or moderate and included (≥10% of patients) dysuria (41%), pollakiuria (21%), hematuria (16%), micturition urgency (14%), urinary tract infection (14%), and fatigue (11%). One death occurred but was not deemed related to treatment.
Thus, Dr. Huang concluded that primary chemoablation of LG IR NMIBC using UGN-102 results in a high rate of disease eradication with encouraging durability and may provide an alternative to repetitive surgery for these patients. These preliminary data form the basis for an ongoing phase 3, randomized, controlled trial of UGN-102 for treatment of LG IR NMIBC
Presented by: William Huang, MD, NYU Langone, New York, NY
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


