(UroToday.com) As a follow-up to the JAVELIN BLADDER 100 trial,1 which demonstrated the efficacy of avelumab as first-line maintenance therapy for patients with advanced urothelial carcinoma who have NOT progressed on first-line platinum-based chemotherapy, Dr. Bellmunt and colleagues herein report post hoc analyses in previously unreported clinical and genomic subgroups.
As a reminder, the JAVELIN Bladder 100 was a landmark trial,1 which demonstrated an OS benefit to the use of avelumab as a first-line (1L) maintenance for patients (pts) with advanced urothelial carcinoma (UC) that had not progressed with 1L platinum-based chemotherapy. This was fully reported on Urotoday. Overall survival at 1 year was 71.3% in the avelumab group and 58.4% in the control group (median overall survival, 21.4 months vs. 14.3 months; hazard ratio for death, 0.69; 95% confidence interval [CI], 0.56 to 0.86; P = 0.001).
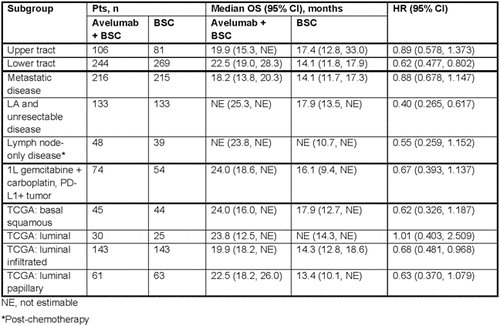
In JAVELIN Bladder 100 (NCT02603432), eligible pts had unresectable locally advanced or metastatic UC without progression with 4-6 cycles of 1L gemcitabine and either cisplatin or carboplatin. The primary endpoint was OS from randomization, assessed in 2 populations: all pts and pts with PD-L1+ tumors (Ventana SP263). In this exploratory analysis, the authors analyzed OS in disease stage and site subgroups, in pts with PD-L1+ tumors who received 1L gemcitabine and carboplatin, and in genomic subtypes (RNAseq whole-transcriptome profiling of tumor tissue) defined using data from The Cancer Genome Atlas (TCGA 2017). Interaction tests were not performed.
Baseline characteristics according to subgroups is summarized below:

Jumping straight into the results of the exploratory analysis, prolonged OS was observed in the avelumab+BSC arm vs the BSC alone arm in pts with upper or lower tract tumors, metastatic or locally advanced (LA) and unresectable disease (prior to chemotherapy), and lymph node-only disease post-chemotherapy (Table).
Forest plots of OS and PFS stratified by the various subgroups is seen below:


OS was also prolonged with avelumab+BSC in pts in PD-L1+ tumors who had received 1L gemcitabine and carboplatin, consistent with findings in the overall population. The KM curve is seen below:

In genomic subtypes, the OS benefit for avelumab+BSC was apparent across TCGA subtypes except luminal subtype.
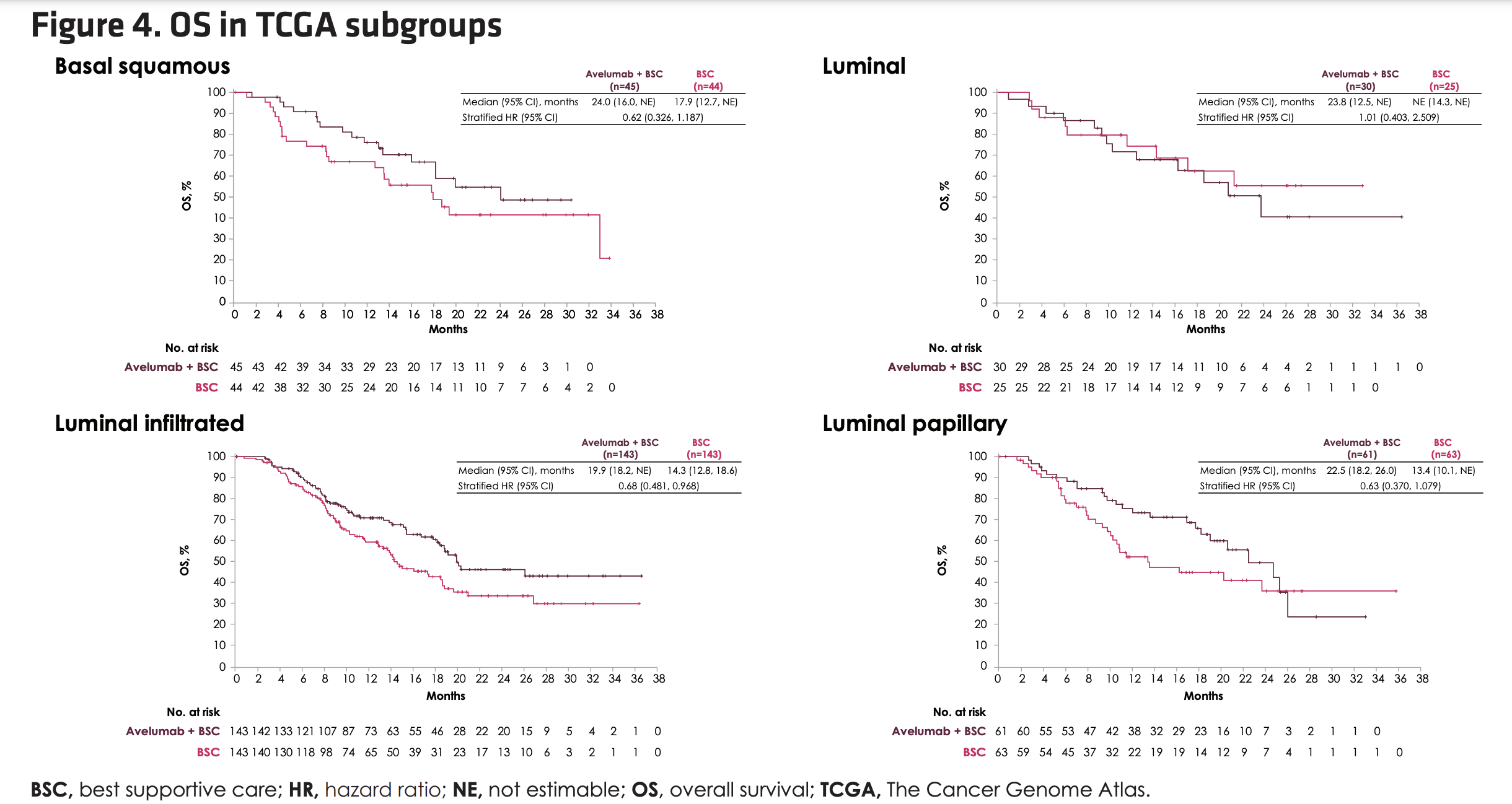
However, an analysis of biomarkers by TCGA subtype did not predict benefit to 1L avelumab maintenance.
Based on this, the authors conclude that OS benefit was seen for avelumab 1L maintenance+BSC vs BSC alone across subgroups of interest. These results are consistent with previously reported findings, further supporting avelumab 1L maintenance as a standard of care for pts with advanced UC that have not progressed with 1L platinum-containing chemotherapy.
Presented by: Joaquim Bellmunt, MD, PhD, Associate Professor, Medicine, Harvard Medical School, Director, Bladder Cancer Program , Beth Israel Deaconess Medical Center
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.
References: