(UroToday.com) The American Urologic Association (AUA) annual meeting’s evolving landscape of advanced prostate cancer treatment session included a talk by Dr. Joshua Lang discussing the role of chemotherapy and treatment sequencing in patients with mCRPC. Dr. Lang notes that there are a multitude of agents available, with many different sequence options:

Dr. Lang started by discussing sipuleucel-T, based on the findings of the IMPACT trial.1 This trial enrolled 512 patients with mCRPC who had asymptomatic disease/minimally symptomatic with no visceral metastases, randomizing men to three infusions of sipuleucel-T (n=341) or placebo (n=171). The IMPACT trial noted a 4.1-month improvement in OS for those taking sipuleucel-T compared to placebo and a 22% reduction in risk of death. There was no difference between the groups with regards to objective disease progression or PSA response. Dr. Lang notes that his key take-away points for sipuleucel-T are: (i) that sipuleucel-T is best for asymptomatic or minimally symptomatic mCRPC, (ii) the overall survival curves in IMPACT separate after 6 months, (iii) significant PSA decline rate was ~3%, (iv) there was no improvement in time to progression, and (v) there were very few side effects (ie. chills, pyrexia).
With regards to abiraterone, there are several patient comorbidities that require caution such as (i) edema, heart failure, or fluid retention, (ii) severe hypertension, (iii) hepatic dysfunction, active viral hepatitis, or heavy alcohol consumers, (iv) uncontrolled hyperglycemia (from prednisone), (v) wound healing concerns (from prednisone), and (vi) active infections (from prednisone). Patient comorbidities that require caution with the utilization of enzalutamide include (i) prior history of seizures, (ii) history of brain metastases, (iii) patient on medications that can lower the seizure threshold (ie. buproprion), (iv) history of falls, (v) history of strokes, (vi) history of dementia (vii) significant fatigue, and (viii) advanced age (>70 years of age).
A previous randomized, open-label, phase 2, crossover trial has previously aimed to determine the best sequence in which to use enzalutamide and abiraterone.2 Patients were randomly assigned to abiraterone acetate 1000 mg orally once daily plus prednisone 5 mg orally twice daily until PSA progression followed by crossover to enzalutamide 160 mg orally once daily (n=101; group A) versus the opposite sequence (n=101; group B). Time to second PSA progression was longer in group A than in group B (median 19.3 months, 95% CI 16.0-30.5 vs 15.2 months, 95% CI 11.9-19.8 months; HR 0.66, 95% CI 0.45-0.97), at a median follow-up of 22.8 months:
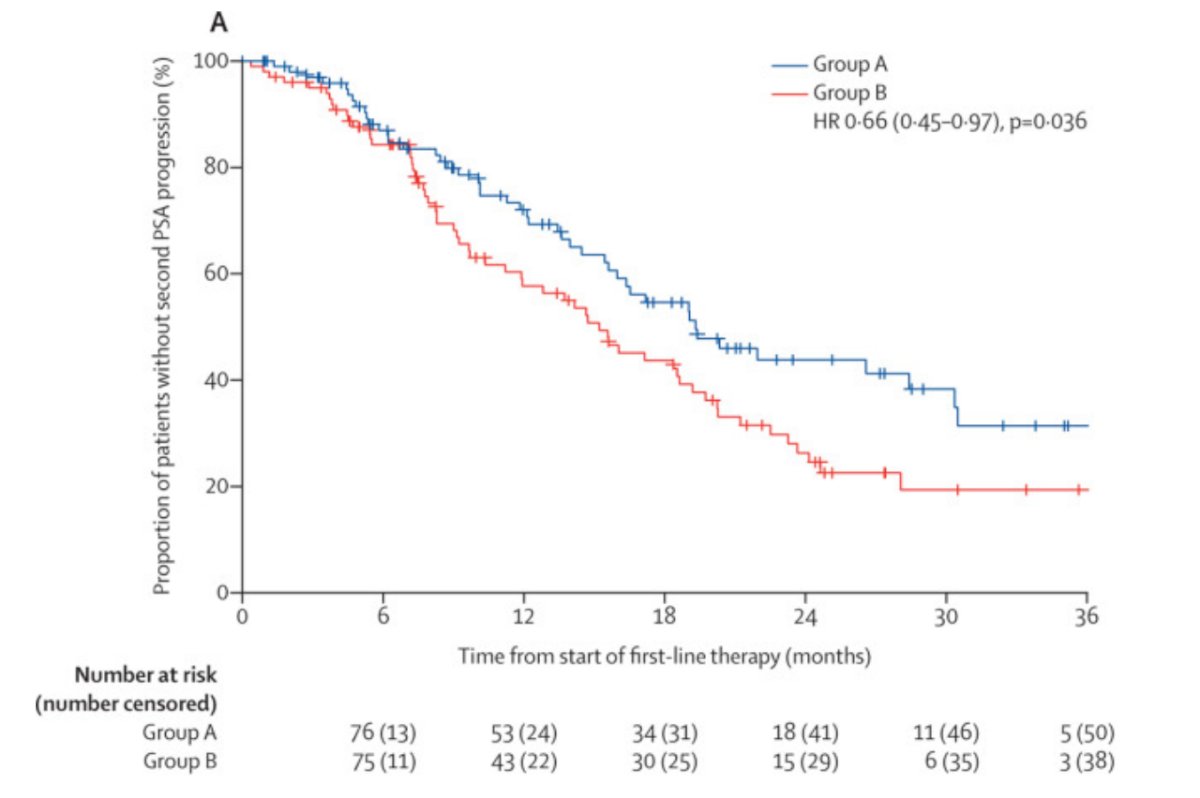
Dr. Lang poses the question ‘which treatment modality should go first?’ Importantly, there are toxicity considerations, such as hepatic dysfunction, fluid excess, and hyperglycemia for abiraterone, and seizures, elderly, and fatigue for enzalutamide. Additionally, there are unique situations with rapid disease progression or significant symptoms and/or visceral disease in which consideration should be given to docetaxel, docetaxel/carboplatin for aggressive variants, and/or etoposide/cisplatin for neuroendocrine/small cell carcinoma.
When considering the time of radium-223 and docetaxel chemotherapy, Dr. Lang notes that it is important to assess data from the ALSYMPCA trial. A prespecified subgroup analysis was undertaken to assess the effect of previous docetaxel use on the efficacy and safety of radium-223.3 In this analysis, there were 526 (57%) of 921 randomly assigned patients had received previous docetaxel treatment (352 in the radium-223 group and 174 in the placebo group) and 395 (43%) had not (262 in the radium-223 group and 133 in the placebo group). Radium-223 prolonged median overall survival compared with placebo, irrespective of previous docetaxel use (previous docetaxel use, HR 0.70, 95% CI 0.56-0.88; no previous docetaxel use, HR 0.69, 0.52-0.92):
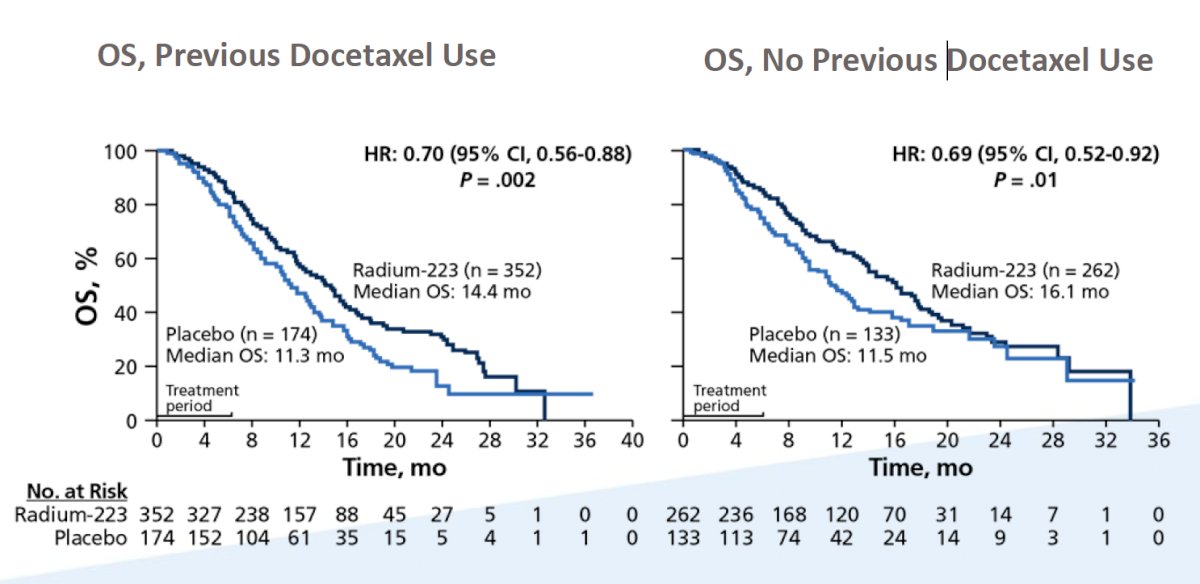
When considering whether radium-223 should be used before or after chemotherapy, there are several important considerations:
- Radium-223 is the only FDA approved agent for patients lacking visceral metastasis
- There are stringent eligibility requirements for treatment, including an initial ANC >=1,500/L with subsequent >= 1,000/L
- Hemoglobin >= 10 g/dL
- Platelets >=100,000/lL with subsequent >= 50,000/L
- Radium-223 requires pre-authorization, while chemotherapy with docetaxel does not
- Patients are more likely to be able to tolerate all six doses of Radium-223 in the pre- versus the post-chemotherapy setting
Given that docetaxel was the first drug to improve overall survival in mCRPC based on the SWOG 99-16 and TAX-327 trials, Dr. Lang highlights several aspects that make a patient ideal for docetaxel. Patients should receive docetaxel who are symptomatic from mCRPC, have mCRPC with visceral metastases (particularly liver metastases), those with rapidly progressive disease (ie. PSA and radiographic progression), men that have had at least first-line 2nd generation androgen pathway inhibitor therapy, and men with metastatic hormone-sensitive prostate cancer (among other agents that are approved – enzalutamide, apalutamide, abiraterone).
Cabazitaxel gained prominence in the second-line mCRPC setting with the reporting of the TROPIC trial in 2010.4 mCRPC patients that had previously received docetaxel were randomized to cabazitaxel versus mitoxantrone with a primary outcome of overall survival. At the cutoff for the final analysis, median survival was 15.1 months (95% CI 14.1-16.3) in the cabazitaxel group and 12.7 months (11.6-13.7) in the mitoxantrone group, with a hazard ratio for death of cabazitaxel versus mitoxantrone 0.70 (95% CI 0.59-0.83). The most common clinically significant grade 3 or higher adverse events were neutropenia (cabazitaxel 82% of patients vs mitoxantrone 58%) and diarrhea (cabazitaxel 6% vs <1%).
Dr. Lang then discussed the PROSELICA trial,5 which tested cabazitaxel 25 mg/m2 versus cabazitaxel 20 mg/m2 among men with mCRPC who had progressed on docetaxel to test the noninferiority of cabazitaxel 20 mg/m2 given the high incidence of side-effects among patients receiving cabazitaxel 25 mg/m2. Overall, 1,200 patients were randomly assigned, including 598 to cabazitaxel 20 mg/m2 and 602 to cabazitaxel 25 mg/m2. Median OS was 13.4 months for cabazitaxel 20 mg/m2 and 14.5 months for cabazitaxel 25 mg/m2 (non-inferior HR of 1.024):
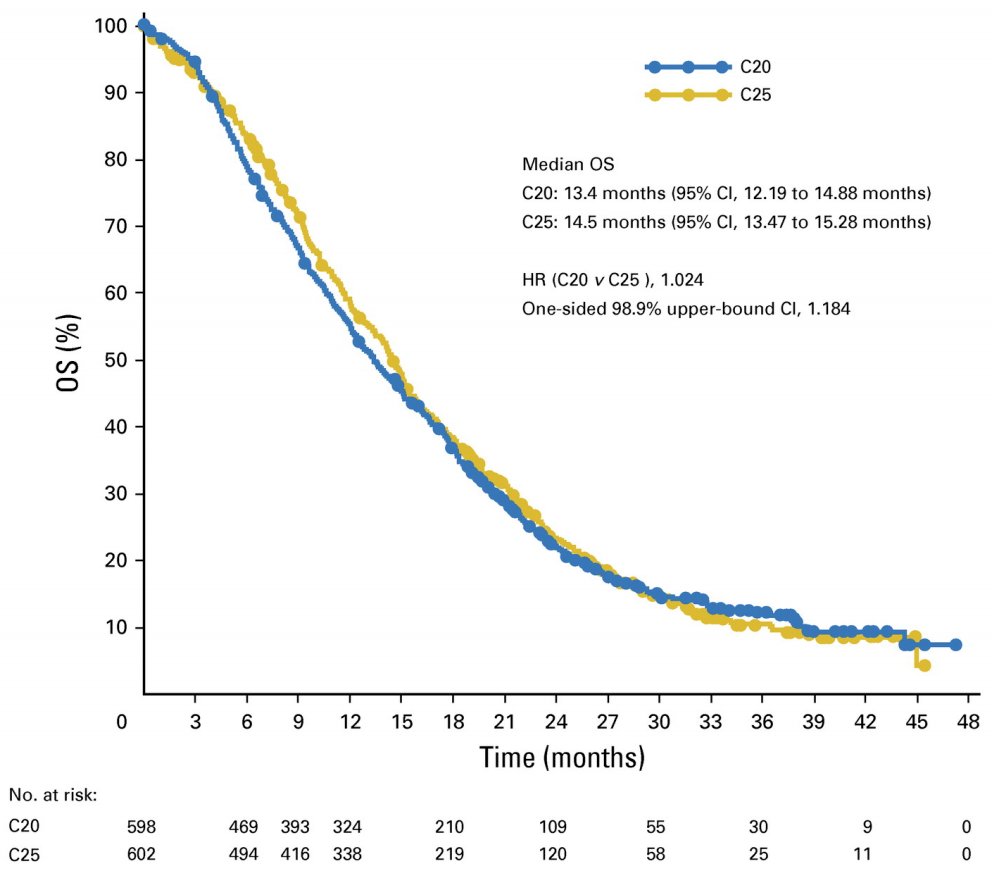
Importantly, the rates of grade 3 or 4 treatment-emergent adverse events were 39.7% for cabazitaxel 20 mg/m2 and 54.5% for cabazitaxel 25 mg/m2.
The CARD trial demonstrated the superiority of cabazitaxel over abiraterone or enzalutamide in patients with mCRPC who had previously received docetaxel and progressed ≤ 12 months on the alternative androgen receptor-targeted agent.6 Patients receiving cabazitaxel had a median overall survival of 13.6 months compared to 11.0 months for those receiving the alternative androgen-signaling targeted inhibitor (HR 0.64, 95% CI 0.46-0.89):
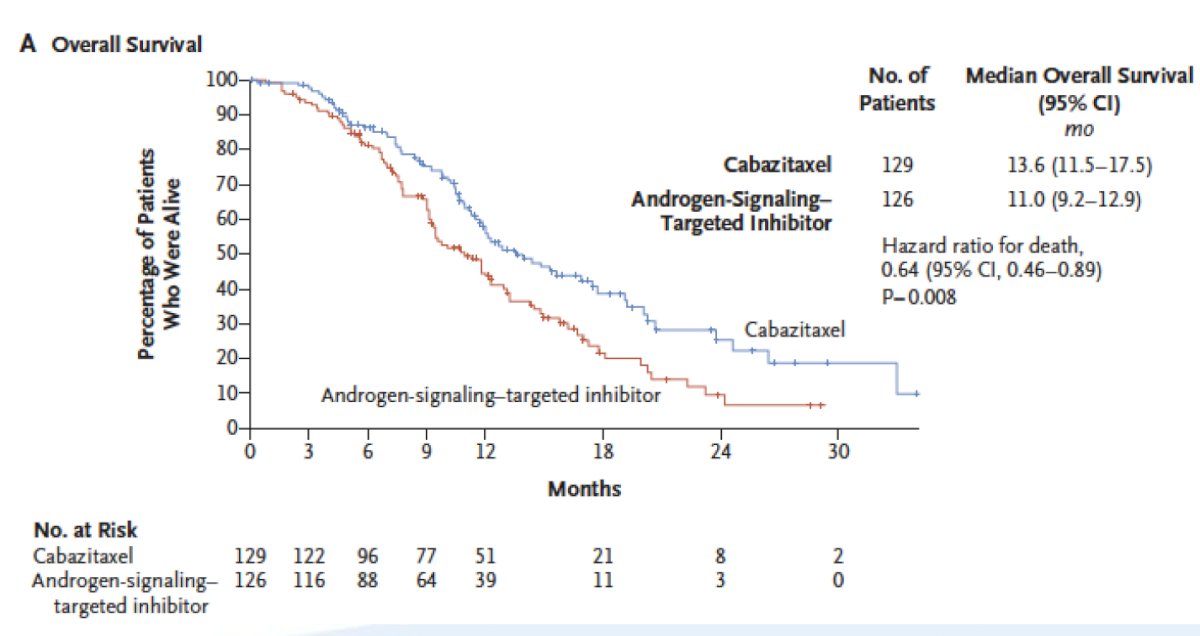
Dr. Lang emphasized that there are important situations in which clinicians should consider a metastatic biopsy to ensure the tumor is still an adenocarcinoma. These include (i) visceral lesions, especially liver metastasis, (ii) extremely bulky lymph nodes (>5 cm), (iii) low PSA in the setting of a very high volume of disease, and (iv) predominantly lytic rather than blastic bone metastases. It is important to note that a biopsy of a metastatic site is required for somatic testing of the tumor.
Neuroendocrine/small cell prostate cancer is a rare diagnosis, with this histologic entity encompassing <1% of new diagnoses. Neuroendocrine/small cell prostate cancer may arise as a mechanism of resistance to ADT, with metastatic disease presenting at unusual sites, with low or modestly rising PSA. Furthermore, elevated CEA or serum neuroendocrine markers (such as chromogranin, neuron-specific enolase, etc) can support the diagnosis, and tissue immunohistochemistry expresses chromogranin A and synaptophysin. Neuroendocrine/small cell prostate cancer is treated like small cell lung cancer and is treated with platinum-doublet chemotherapy such as cisplatin or carboplatin with etoposide.
DNA repair gene alterations are common in metastatic prostate cancer, given that among mCRPC patients 23% harbor DNA repair alterations, with the frequency of DNA repair alterations increasing with disease progression. Among men with metastatic prostate cancer, 11.8% have a germline alteration in 16 DNA damage repair genes, with age and family history not influencing mutation frequency.7 Dr. Lang then discussed several key trials in the approval of PARP inhibitors in mCRPC patients. The TOPARP trial was an investigator-initiated, open-label, multi-stage phase 2 study with an adaptive design focused on predictive biomarker identification. Part A was a test set of all comers, and TOPARP-A demonstrated that treatment with the PARP inhibitor olaparib was associated with improvements in radiographic progression-free survival and overall survival, specifically among patients with extensively pre-treated mCRPC who had DNA-repair defects:8 16 of 49 patients (33%) had a response to olaparib, with 12 patients remaining on treatment for >6 months.
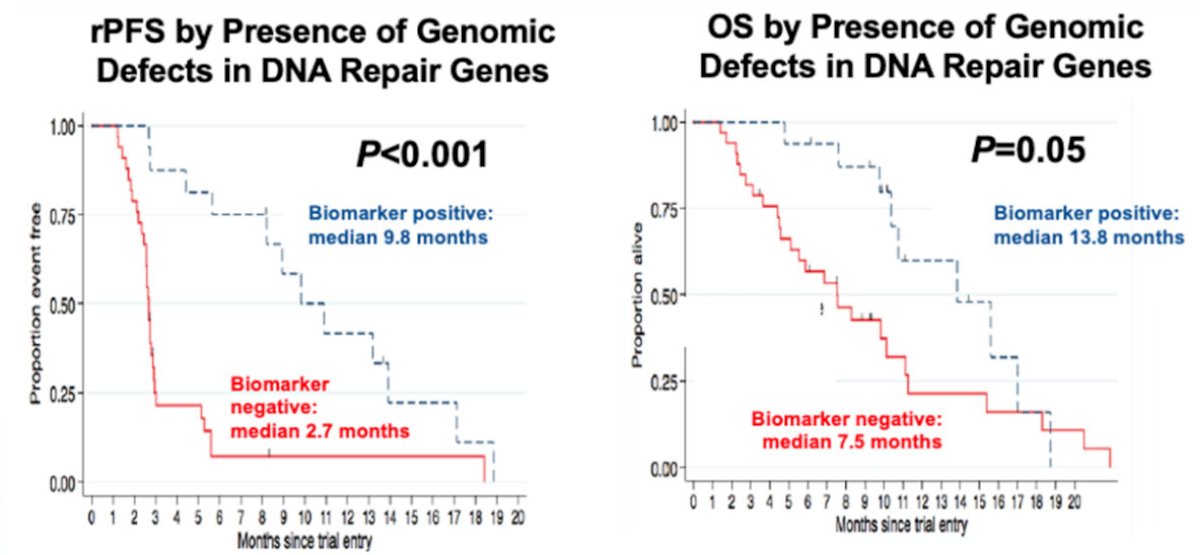
The PROfound study was a phase III trial of men with mCRPC who had progressed on previous abiraterone acetate or enzalutamide.9 The investigators used the FoundationOne CDx assay to identify alterations in one of 15 pre-specified genes involved in homologous recombination repair (BRCA 1/2, ATM, BRIP1, BARD1, CDK12, CHEK 1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L). Cohort A included patients with alterations in BRCA1, BRCA2, or ATM while Cohort B had alterations in any of the other 12 included genes. In both cohorts, patients were randomized 2:1 to olaparib vs. abiraterone or enzalutamide. The primary analysis was based on imaging-based progression-free survival (soft-tissue according to RESIST 1.1 and bony according to PCCTWG3 criteria) among patients in Cohort A. There was a significantly improved progression-free survival in patients with mutations of BRCA1, BRCA2, or ATM (HR 0.34, 95% CI 0.25 to 0.47):
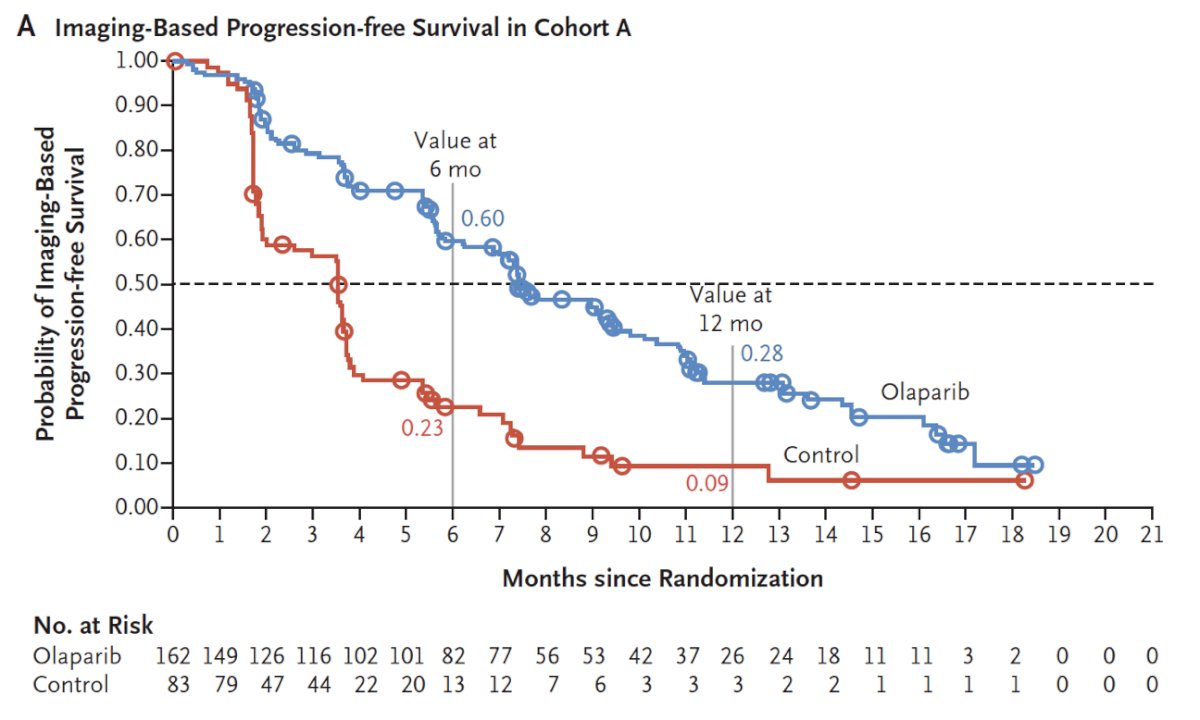
Similar results were seen in the combined cohort (HR 0.49, 95% CI 0.38 to 0.63). Adverse events were common in both patients on olaparib (any = 95%, grade ≥ 3 = 51%) and in the control group (any = 88%, grade ≥ 3 = 38%). An updated analysis also showed a survival benefit for olaparib with a median OS of 19.1 months compared to 14.7 months in the control arm in Cohort A (HR 0.69, 95% CI 0.50-0.97).10
Recently, 177Lu-PSMA-617 has entered the landscape of mCRPC treatment with the publication of the highly touted VISION trial.11 The VISION trial is an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care (SOC) or SOC alone. The trial schema for VISION is as follows:
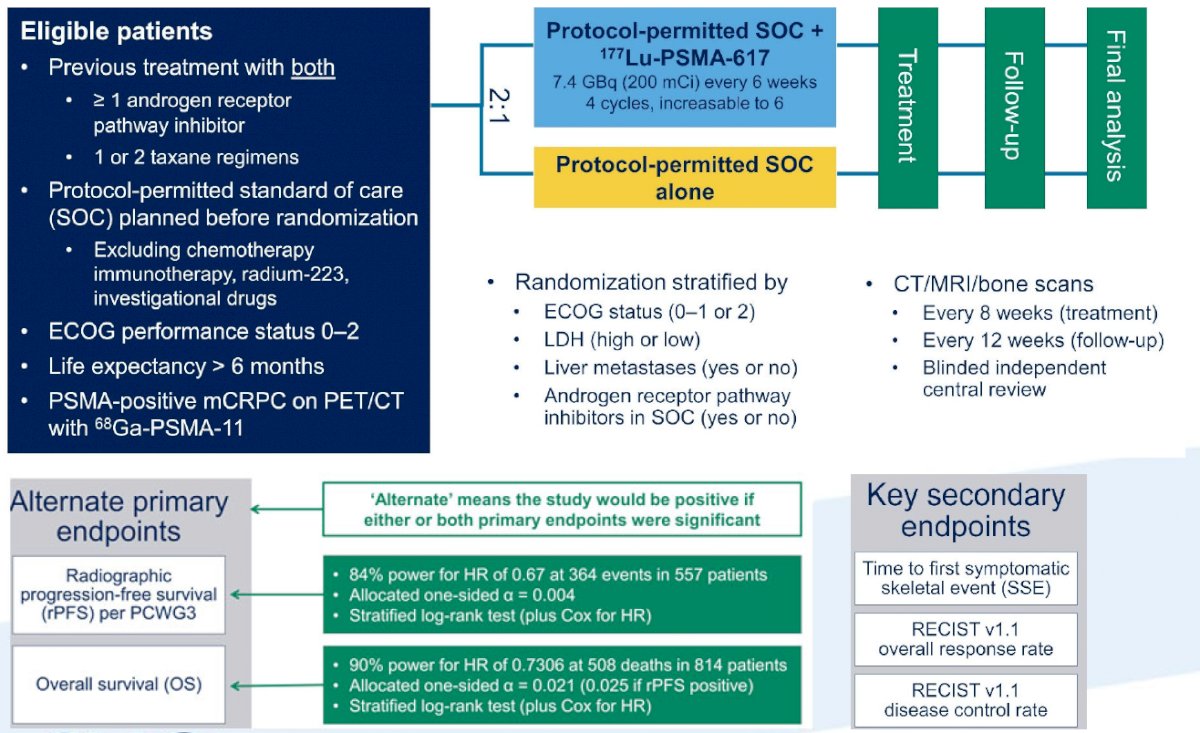
The trial assessed two alternate primary endpoints: rPFS using PCWG3 criteria by independent central review and OS. Among 1,179 screened patients, the VISION trial enrolled 831 patients, including 551 patients were allocated to 177Lu-PSMA-617 + SOC and 280 were allocated to SOC only. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617+ SOC significantly improved overall survival by a median of 4.0 months (median OS, 15.3 vs 11.3 months; HR 0.62, 95% CI 0.52 0.74), compared to SOC alone, in the overall cohort of all randomized patients (n=831).
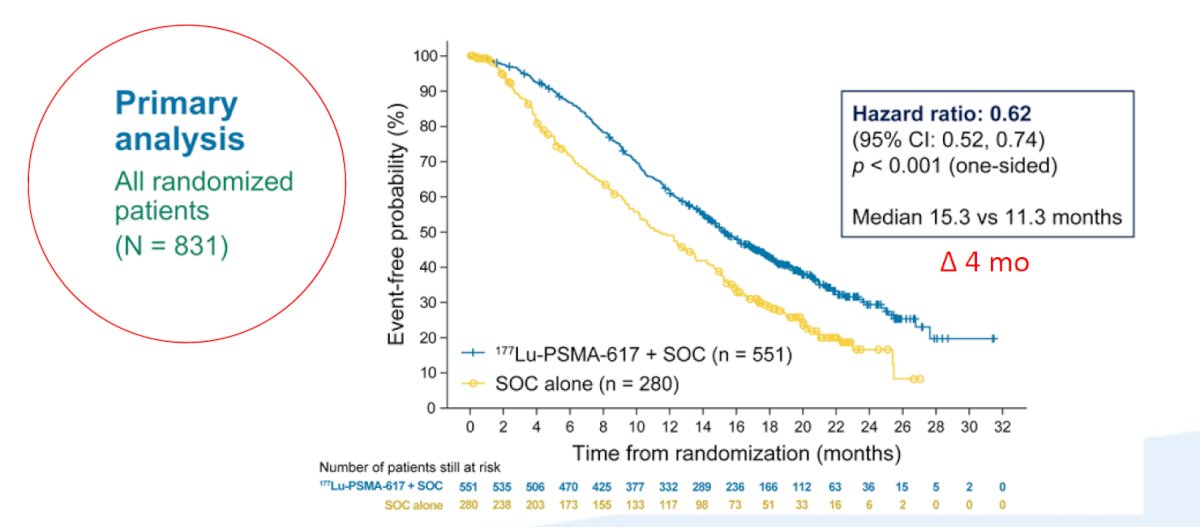
The second alternate primary endpoint showed that treatment with 177Lu-PSMA-617 + SOC significantly improved rPFS by a median 5.3 months (median rPFS, 8.7 vs 3.4 months; HR 0.40, 99.2% CI 0.29 0.57):
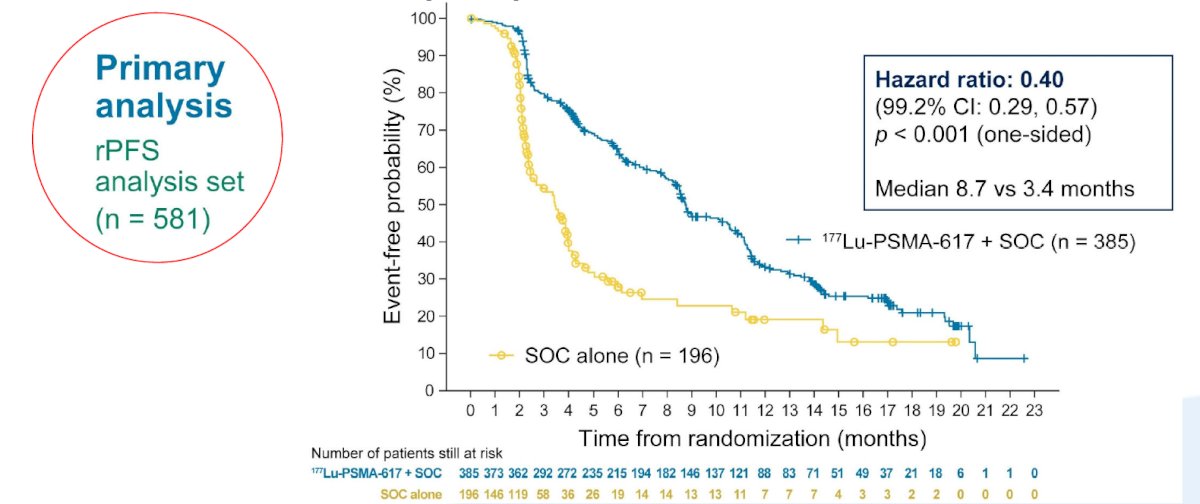
While a higher rate of high-grade (grade 3-5) treatment-emergent adverse events was observed with 177Lu-PSMA-617 (28.4% vs 3.9%), overall therapy was well tolerated. In terms of specific adverse events, treatment with 177Lu-PSMA-617+ SOC was associated with increased rates of bone marrow suppression, xerostomia, and nausea and vomiting.
Dr. Lang concluded his presentation with several summary points:
- There are five classes of FDA approved therapies that improve survival for men with mCRPC
- Try to use sipuleucel-T early in the disease process
- Sequencing of abiraterone and enzalutamide does not have outstanding response rates for the second agent
- Radium-223 should be utilized when possible prior to docetaxel for practical reasons
- Cabazitaxel should be dosed at 20 mg/m2 IV every three weeks
- Germline and somatic testing of patients with CRPC is required for PARP inhibitor eligibility
- Platinum doublet chemotherapy should be considered for neuroendocrine/small cell carcinoma and those with homologous recombination deficiency
- Future treatment strategies will likely incorporate PARP inhibitor combinations, PD-(L)1 antibody, and PSMA-targeted theranostics
Presented by: Joshua M. Lang, MD, MS, Associate Professor of Medicine, Division of Hematology/Oncology/Palliative Care, University of Wisconsin-Madison, Madison, WI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.
References:
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363(5):411-422.
- Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomized, open-label, phase 2, crossover trial. Lancet Oncol. 2019 Dec;20(12):1730-1739.
- Hoskin P, Sartor O, O’Suillivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and asymptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomized, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014 Nov;15(12):1397-1406.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010;376(9747):1147-1154.
- Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol 2017 Oct 1;35(28):3198-3206.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019 Dec 26;381(26):2506-2518.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. The New England journal of medicine. 2015;373(18):1697-1708.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Dec 10;383(24):2345-2357.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Jun 23 [Epub ahead of print].


