(UroToday.com) The treatment landscape in advanced prostate cancer has rapidly evolved. In particular, agents with demonstrated survival benefits later in the natural history of prostate cancer have been used earlier in the disease process. In the context of metastatic castration-sensitive prostate cancer (mCSPC), docetaxel was the first agent to demonstrate a survival benefit when combined with conventional androgen deprivation therapy (ADT). Following this, a number of androgen receptor targeting agents have been assessed, beginning with abiraterone acetate. In TITAN, the addition of apalutamide to ADT has demonstrated improvement in long-term outcomes for patients with mCSPC.
In a moderated poster presentation at the American Urologic Association Virtual Annual Meeting, the relationships between the number and location of metastases and signatures associated with poor prognosis and ADT insensitivity and outcome measures were assessed.
While previously published, to briefly summarize, TITAN enrolled 1052 patients with mCSPC who had at least 1 bone lesion. Enrolled patients were randomized 1:1 to apalutamide (240 mg QD) or placebo (PBO)+ADT, and were stratified by sites of metastases.
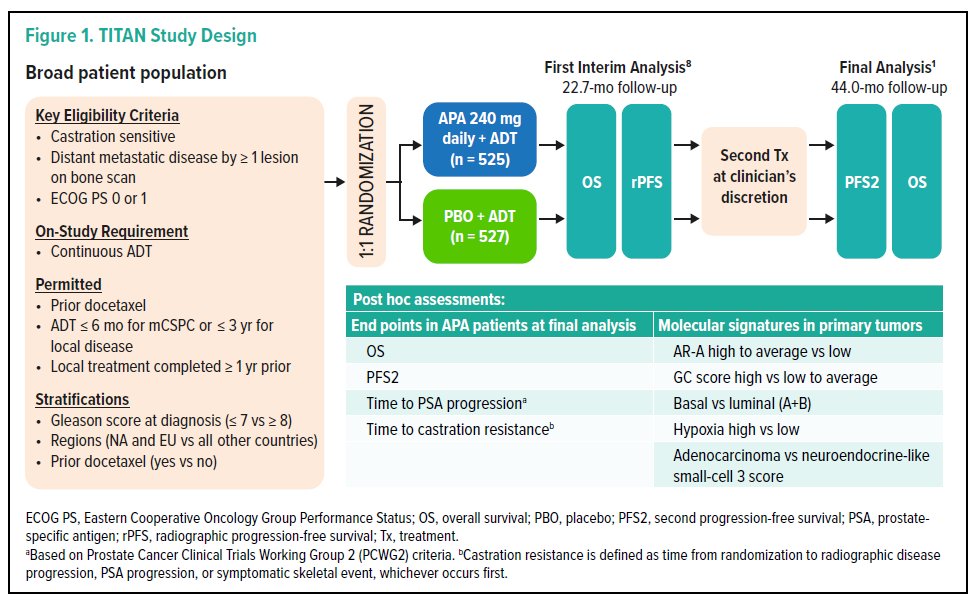
The authors examined outcomes in apalutamide-treated pts (n=525) using Kaplan-Meier and Cox proportional hazards methods. Further, they defined molecular subtypes from 222 primary tumors (biomarker population; apalutamide =110, PBO=112) using biologically distinct signatures categorized by expression levels: androgen receptor activity (AR-A) high to average vs low, genomic classifier (GC) score high vs low to average, basal vs luminal (A + B), hypoxia high vs low, and adenocarcinoma vs neuroendocrine-like small-cell 3 scores. The association between categorical expression and sites of metastases was assessed with Fisher’s exact test.
The distribution of metastatic sites at baseline among the 1052 enrolled patients were as follows: 53.0% in bone only, 37.9% in bone +1 other location, 9.0% in bone +2 or 3 other locations; 38.3% of pts had >10 bone lesions. An increasing number of lesions was associated with worse outcomes among patients receiving apalutamide.
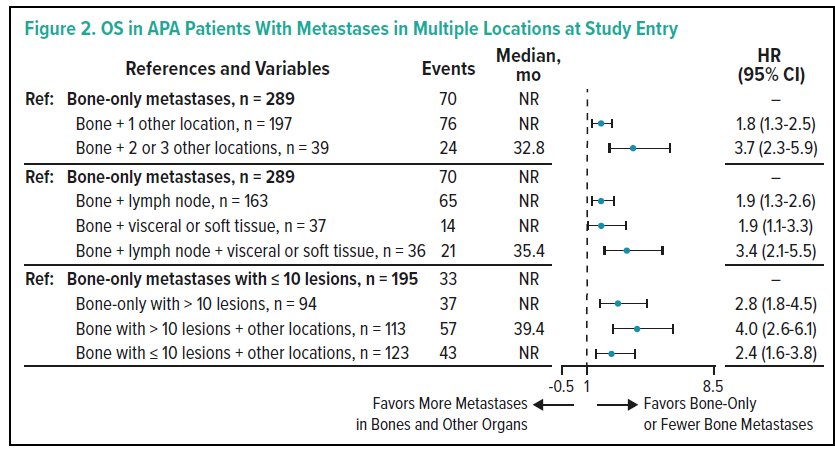
Further, patients with multiple metastatic locations at BL have a significantly higher risk of worse outcomes compared to those with bone-only metastases.
In the biomarker analysis, the authors found that 43.2% of patients had AR-A low, 74.8% GC high, 50.5% basal, 50.0% hypoxia low, and 83.8% adenocarcinoma subtypes. Primary tumors from patients with bone-only metastases were significantly enriched for low levels of hypoxia or adenocarcinoma while those with concomitant bone and visceral metastases were significantly enriched for basal, AR-A low, or neuroendocrine-like features, respectively (p<0.05 for all).
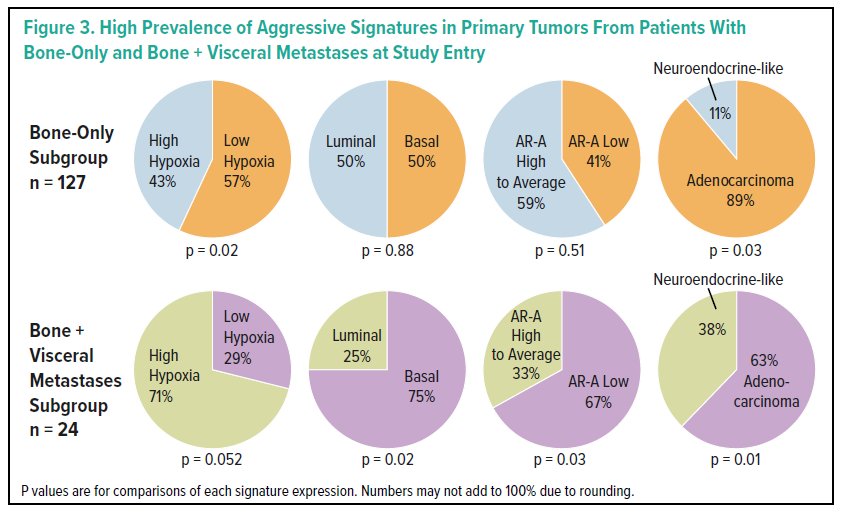
The authors then concluded that, among patients receiving apalutamide for mCSPC in the TITAN trial, those with multiple anatomic locations and >10 bone metastases had significantly worse oncologic outcomes. Further, metastatic location was preliminarily associated with specific phenotypes.
Presented by: Anders Bjartell, Professor at Urological cancer, Malmö, Lund University
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


