(UroToday.com) The treatment landscape in advanced prostate cancer has rapidly evolved. In the context of metastatic castration resistant prostate cancer (mCRPC), docetaxel was the first agent to demonstrate a survival benefit when combined with conventional androgen deprivation therapy (ADT). Following this, a number of androgen receptor targeting agents have been assessed, beginning with abiraterone acetate. More recently, targeted therapies including PARP inhibitors and immunotherapy have been assessed. In the phase 1/2 KEYNOTE-365 study (NCT02861573), the combination of pembrolizumab (an anti-PD-1 checkpoint inhibitor) and olaparib (a PARP inhibitor) demonstrated antitumor activity and acceptable safety in molecularly unselected patients treated with docetaxel for mCRPC (cohort A).
In a moderated poster presentation at the American Urologic Association Virtual Annual Meeting, Dr. Nordquist and colleagues presented updated results from cohort A with a minimum of 11.4 months of follow-up.
Cohort A of KEYNOTE-365 enrolled patients with molecularly unselected, docetaxel-pretreated mCRPC whose disease progressed within 6 months of screening. Patients received pembrolizumab 200 mg intravenously every 3 weeks + olaparib 400 mg capsule or 300 mg tablet orally twice daily. The primary endpoints included prostate-specific antigen (PSA) response rate (PSA decrease of ≥50% from baseline), objective response rate (ORR) per RECIST v1.1 by blinded independent central review (BICR), and safety. Further, secondary endpoints were time to PSA progression, disease control rate (DCR), duration of response (DOR), radiographic progression-free survival (rPFS), and overall survival (OS).
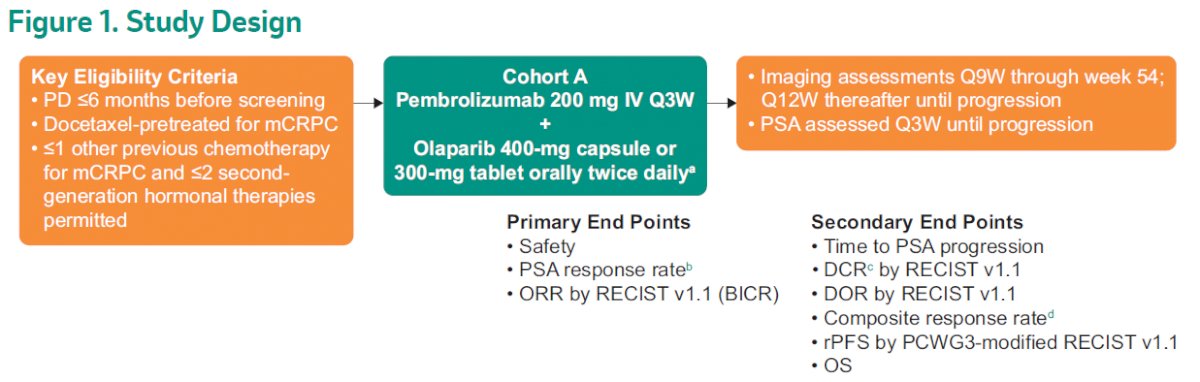
With a data cutoff of November 12, 2020, the median follow-up time from enrollment to data cutoff was 19.3 months (range 11.4-45.9). Among 104 initially enrolled patients, 102 were treated and 92 patients discontinued treatment, primarily because of progressive disease (51.0%). In terms of baseline characteristics, the median age was 69.5 years (range 47-84), 28.4% of patients were PD-L1+, 33.3% had evidence of visceral disease, and 56.9% had measurable disease per BICR.
In terms of the primary outcomes, the confirmed PSA response rate in all patients with a PSA measurement at baseline (N=102) was 14.7% (95% CI 8.5-23.1).
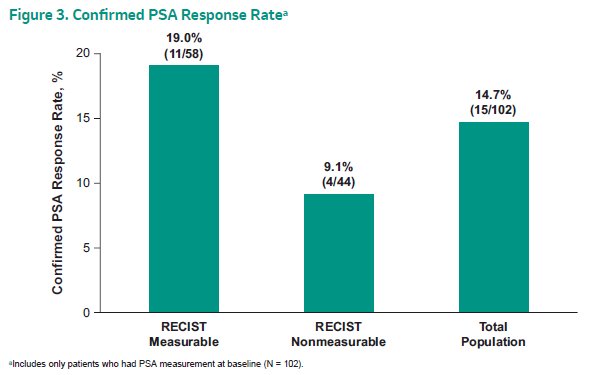
The median (95% CI) time to PSA progression was 4.0 months (3.0-4.9 months). Among the 58 patients with measurable disease at baseline, the confirmed ORR was 6.9% (1.9-16.7%; 4 partial responses). The median DOR was not reached (range 7.2+ to 37.8+ months). Notably, 2 patients had a response that exceed 12 months.
In all 102 treated patients, the DCR was 26.5% (95% CI 18.2-36.1). The median (95% CI) rPFS was 5.2 months (4.1-6.5) and median OS was 14.4 months (10.4-17.9).
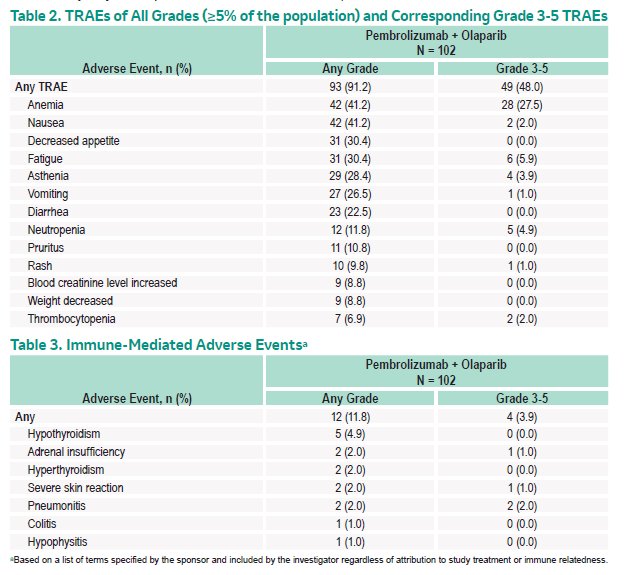
In terms of safety, treatment-related adverse events (TRAEs) occurred in 93 patients (91.2%), the most frequent of which (≥30%) were anemia (41.2%), nausea (41.2%), decreased appetite (30.4%), and fatigue (30.4%). While many adverse events were minor, grade 3-5 TRAEs occurred in 49 patients (48.0%) and six patients (5.9%) dying of adverse events (of which, 2 were treatment-related).
The authors concluded that, with this longer duration of follow-up, the combination of pembrolizumab and olaparib continued to show some antitumor activity in patients with molecularly unselected, docetaxel-pretreated mCRPC.
Presented by: Luke T. Nordquist, MD, Urology Cancer Center, Omaha, Nebraska
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


