(UroToday.com) The treatment landscape in advanced prostate cancer has rapidly evolved. In the context of metastatic castration resistant prostate cancer (mCRPC), docetaxel was the first agent to demonstrate a survival benefit when combined with conventional androgen deprivation therapy (ADT). Following this, a number of other treatment approaches have been assessed and are now clinically used including androgen receptor targeting agents. Additionally, agents with demonstrated survival benefits later in the natural history of prostate cancer have been used earlier in the disease process, including both metastatic castration sensitive prostate cancer (mCSPC) and non-metastatic castration resistant prostate cancer (nmCRPC). The phase 3 TITAN and SPARTAN studies demonstrated improved outcomes with the addition of apalutamide to androgen deprivation therapy (ADT) for patients with mCSPC and nmCRPC, respectively.
In a podium presentation at the American Urologic Association Annual Meeting, Dr. Kim Chi and colleagues presented a post hoc analysis of prostate-specific antigen kinetics (PSA) kinetics in patients enrolled in these two trials.
The design of each of these studies has previously been published. In this post hoc analysis, the authors assessed baseline PSA at randomization, time to PSA nadir, and proportion of patients achieving a PSA decline of ≥90% (PSA90) and of patients achieving a PSA≤0.2 ng/mL at 3 and 12 months and at any time after treatment in the apalutamide arms of the two trials. Within each study, rPFS/MFS were compared between patients achieving a PSA90 or PSA≤0.2 ng/mL response vs not.
The authors included 525 patients treated on TITAN and 806 patients treated on SPARTAN who received apalutamide. Median baseline PSA, time to PSA nadir, median PSA nadir, and maximum percentage changes from baseline PSA are shown in the table. Apaluatmide + ADT was associated with both rapid and profound declines in PSA in both trials.
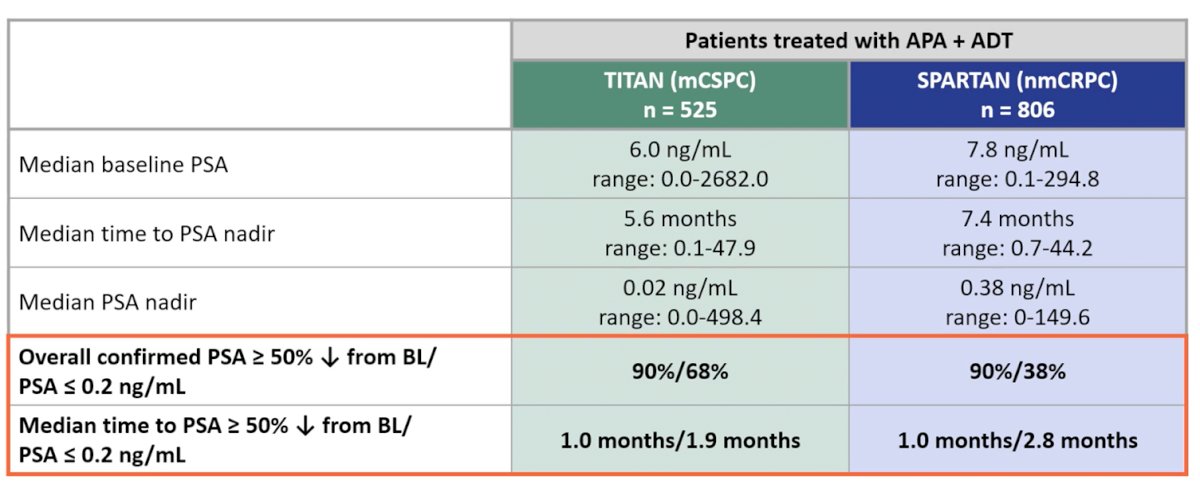
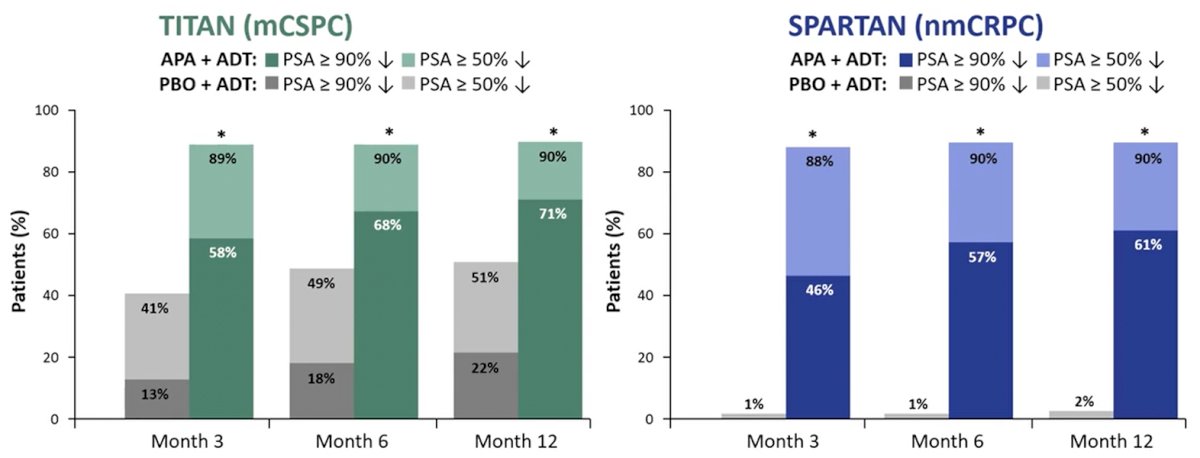
PSA90 and confirmed PSA≤0.2 ng/mL were evident as early as 3 months in both TITAN and SPARTAN populations, though the percentage of confirmed response continued to increase at 12 months. These declines were substantially more common in patients receiving apalutamide + ADT as compared to ADT alone.
Prognostically, patients treated with apalutamide who achieved PSA90 were at lower risk of rPFS events in TITAN and of MFS events in SPARTAN, with a hazard ratio (95% confidence interval) of 0.46 (0.321-0.653) and 0.36 (0.271-0.489) in each respective study (both p<0.0001), compared with patients receiving apalutamide who did not achieve PSA90. Patients with confirmed PSA≤0.2 ng/mL had similar rPFS and MFS benefits.
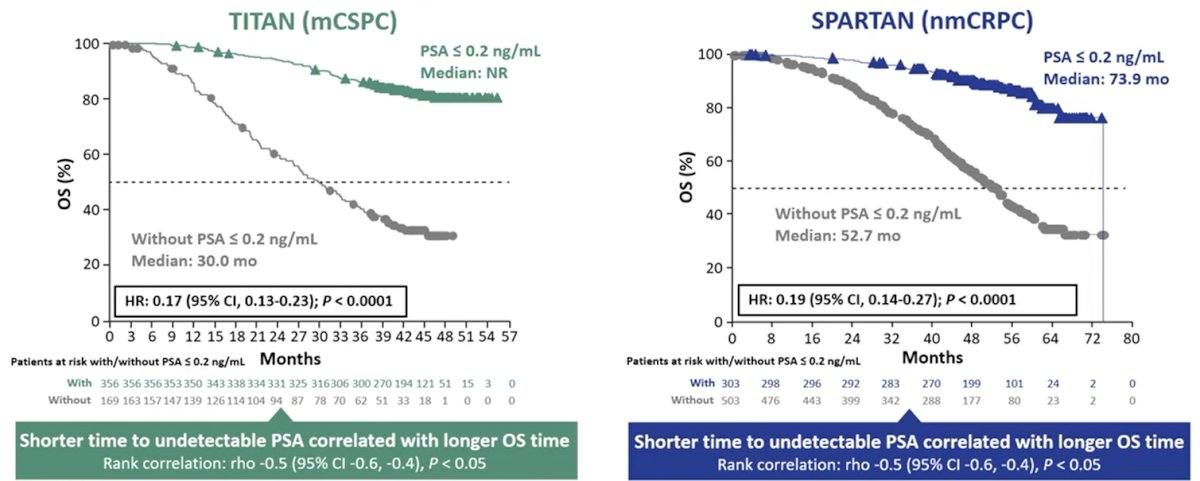
Dr. Chi concluded that patients with advanced PC, whether mCSPC or nmCRPC, who received apalutamide +ADT demonstrated rapid PSA declines that continued over time. There was a high rate of patients with PSA90 and with PSA≤0.2 ng/mL responses, with a majority of patients reaching PSA90 by 12 months. Those patients who had PSA90 and/or PSA nadir of ≤0.2 ng/mL had improved long-term oncologic outcomes.
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


