(UroToday.com) The treatment landscape in advanced prostate cancer has rapidly evolved. In the context of metastatic castration resistant prostate cancer (mCRPC), docetaxel was the first agent to demonstrate a survival benefit when combined with conventional androgen deprivation therapy (ADT). Following this, a number of other treatment approaches have been assessed and are now clinically used including androgen receptor targeting agents. Additionally, agents with demonstrated survival benefits later in the natural history of prostate cancer have been used earlier in the disease process, including both metastatic castration sensitive prostate cancer (mCSPC) and non-metastatic castration resistant prostate cancer (nmCRPC). In the phase III ARAMIS trial (NCT02200614), darolutamide significantly reduced the risk of metastasis and death vs placebo in patients with nmCRPC.
In a podium presentation at the American Urologic Association Annual Meeting, Dr. Neal Shore and colleagues assessed the relationship between prostate-specific antigen (PSA) response and urinary and bowel adverse events (AEs), time to deterioration in quality of life (QoL), and prostate cancer (PC)–related invasive procedures in ARAMIS.
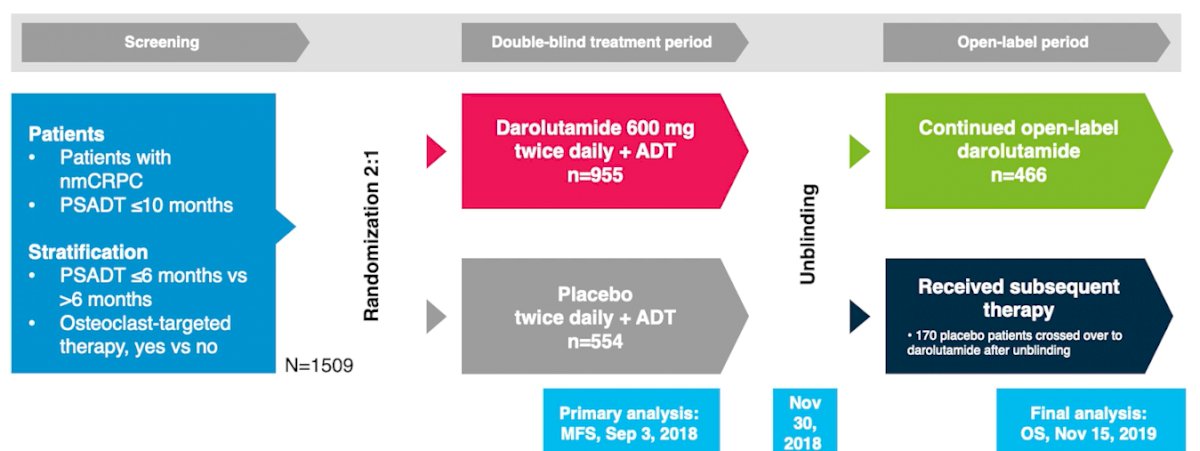
To briefly summarize the previously published ARAMIS trial, men with nonmetastatic castration-resistant prostate cancer were randomized 2:1 to darolutamide (n=955) or placebo (n=554) while continuing androgen deprivation therapy.
In this analysis, the authors examined the proportion of darolutamide patients achieving declines in PSA from baseline to week 16 of >90%, 50%–90%, and <50% and correlated these with urinary and bowel AEs. Time to deterioration in QoL using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-PR25) subscales and Functional Assessment of Cancer Therapy–Prostate, Prostate Cancer Subscale (FACT-P PCS) was defined as first occurrence of a minimally important difference and ≥3 point decline from baseline, respectively, and calculated using Kaplan-Meier estimators and stratified Cox proportional hazard models.
At baseline, prior prostatectomy and radiotherapy were reported for 25.0% and 18.5% of patients randomized to darolutamide and 24.2% and 16.1% of patients randomized to placebo, respectively.
The incidence of urinary AEs showed minimal difference between the treatment groups with respect to urinary tract infection (5.3% vs 5.6%), abnormally frequent urination (4.4% vs 3.2%), and hematuria (4.5% vs 5.4%) but was lower for those receiving darolutamide with respect to urinary retention (3.8% vs 7.4%) and dysuria (2.6% vs 5.2%). Further, among those receiving darolutamide, greater PSA response (>90%, 50%–90% and <50%) was associated with a lower incidence of urinary retention (2.2%, 4.2% and 5.1%) and dysuria (0.5%, 3.2%, 5.1%), respectively.
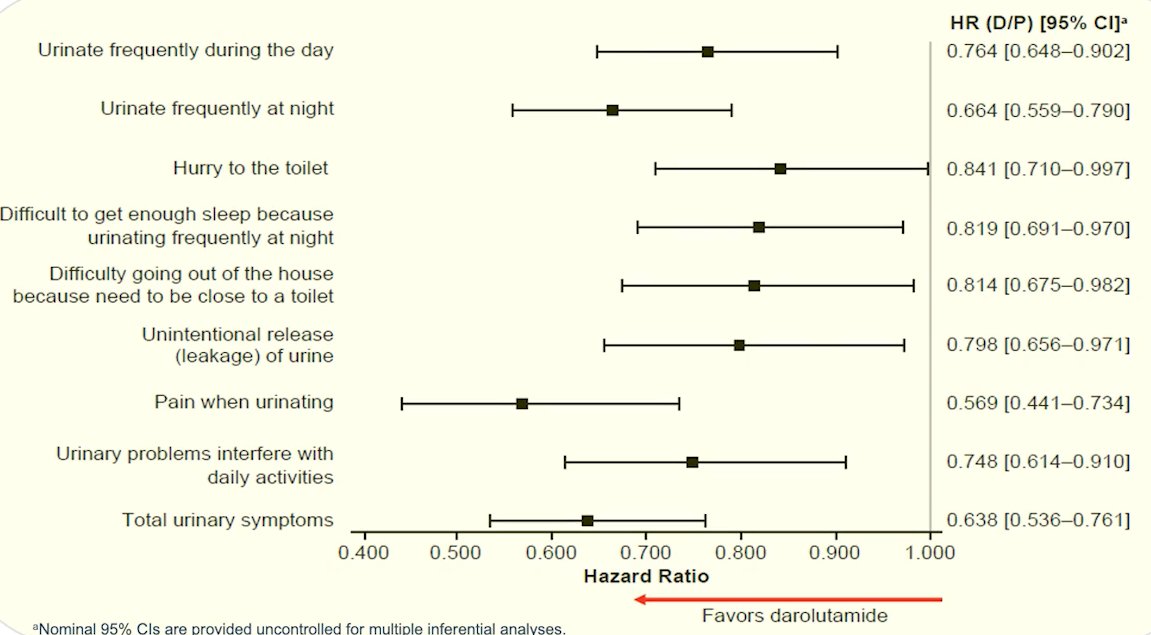
Additionally, darolutamide significantly delayed the time to deterioration in QoL vs placebo for EORTC-QLQ-PR25 subscales of urinary symptoms (25.8 vs 14.8 months; hazard ratio [HR] 0.64; 95% confidence interval [CI] 0.54–0.76; p<0.01) and bowel symptoms (18.4 vs 11.5 months; HR 0.78; 95% CI 0.66–0.92; p<0.01) and FACT-P PCS (11.1 vs 7.9 months; HR 0.80; 95% CI 0.70–0.91; p=0.0005).
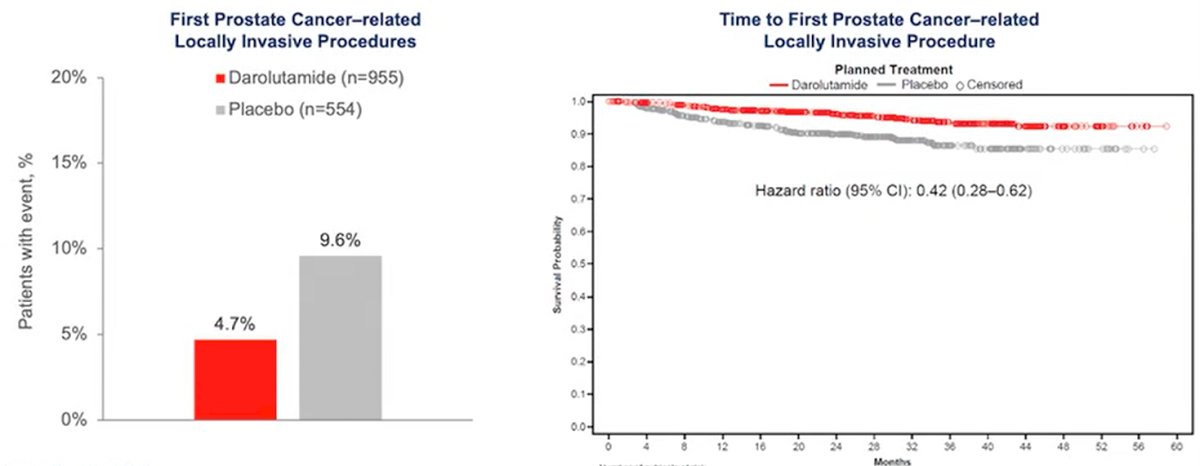
Furthermore, fewer patients receiving darolutamide (4.7%) underwent PC-related invasive procedures compared to those receiving placebo (9.6%). The time to first procedure was, additionally, significantly longer for those receiving darolutamide (HR 0.42; 95% CI 0.28–0.62; p<0.001).
Thus, Dr. Shore concluded that the use of darolutamide in nmCRPC was, in addition to previously proven benefits in metastasis-free survival and overall survival, associated with a reduction in local urinary and bowel symptoms, delayed deterioration in patient QoL related to urinary and bowel symptoms, and reduction in locally invasive procedures.
Presented by: Neal Shore, MD, FACS, is the Medical Director of the Carolina Urologic Research Center. He practices with Atlantic Urology Clinics in Myrtle Beach, South Carolina
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


