(UroToday.com) In a plenary session entitled “Advances in Prostate Cancer: Androgen Deprivation Therapy Across the Disease Continuum” held in conjunction with the American Urologic Association Annual Meeting, Dr. Alicia Morgans explored the role of systemic treatment intensification in metastatic castration sensitive prostate cancer (mCPSC).
She began by highlighting the myriad of treatment options available and guideline-recommended in this disease space including an androgen-deprivation therapy backbone along with apalutamide, abiraterone, docetaxel, or enzalutamide as well as potential local prostate radiotherapy in those with low-volume disease.

Thus, the question becomes how do we choose among the multitude of treatment options. Dr. Morgans emphasized that the choice we make in this disease space will have significant ramifications for downstream treatment options when the patient progresses to castration resistant disease.
To highlight the considerations, she provided an initial case example of a 68-year-old man who presented with progressive fatigue, low back pain, decreased appetite, and weight loss which culminated in an emergency room visit for urinary retention. Laboratory investigations demonstrated a PSA of 144 ng/mL and subsequent prostate biopsy demonstrated Gleason score 5+5 disease with both nodal and bony metastasis evident on conventional imaging.
Dr. Morgans then walked us through treatment options, beginning with chemohormonal therapy. Two studies, CHAARTED and STAMPEDE, demonstrated overall survival benefits with the use of combined docetaxel and androgen deprivation in this patient population. Median overall survival was improved by nearly 14 months in CHAARTED and 10 months in STAMPEDE. A subsequent meta-analysis by Dr. Vale and colleagues demonstrated a 9% absolute improvement in overall survival at 4-years for the use of docetaxel.
The next potential option she discussed was abiraterone acetate. The data for this approach comes from LATITUDE (which was enriched with aggressive disease) and STAMPEDE. In each of these studies, the addition of abiraterone acetate to ADT resulted in significantly improved overall survival, with a relative effect of 37-38%. The TITAN study provided similar data for the use of apalutamide in this disease indication and similarly showed a 33% relative improvement in overall survival, as well as benefits in radiographic progression-free survival.
The final systemic treatment option is enzalutamide. Dr. Morgans first discussed the ARCHES trial which, while similar to the others, assessed the primary endpoint of radiographic progression-free survival. Also, notably, patients who had previously received docetaxel were included. The ENZAMET trial, unlike the others, did not use a control arm of ADT alone but also included a standard non-steroidal antiandrogen. As with ARCHES, docetaxel was allowed though this was included as a planned early/concomitant treatment. Each of these trials was positive with ENZAMET demonstrating improved overall survival with an effect similar to the other trials cited above. Notably, in stratified analyses, those patients who received concomitant docetaxel did not demonstrate survival benefits from the inclusion of enzalutamide while those who did not receive docetaxel had significant improvements.
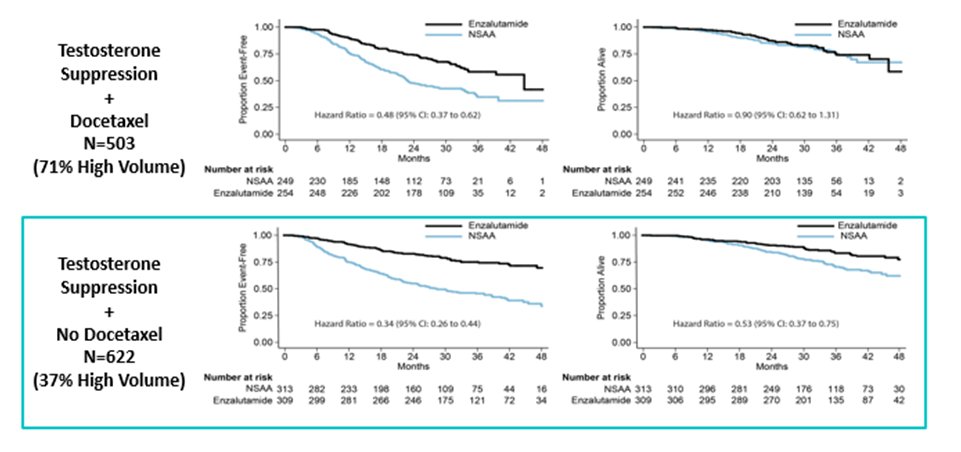
Dr. Morgans then cited some comparative data from STAMPEDE in which patients could have been accrued to the abiraterone or docetaxel treatment arms concurrently. While this was not a direct randomized comparison, the data demonstrate no evidence of differences in overall survival, however, failure-free survival and progression-free survival favoured abiraterone.
Dr. Morgans highlighted than, in choosing our treatment recommendation, we must also consider quality of life effects of therapy and patient preferences. To this end, she reviewed patient-reported quality of life data from each of the studies. In CHAARTED, FACT-P Total scores initially fell for those receiving docetaxel relative to ADT alone (with heavily overlapping 95% confidence intervals) with subsequent later improvements with results overall favourable for treatment with docetaxel given improvements in disease control. In contrast, in LATITUDE, there was a general increase in FACT-P scores for patients on abiraterone, though this did not reach clinically meaningful thresholds.
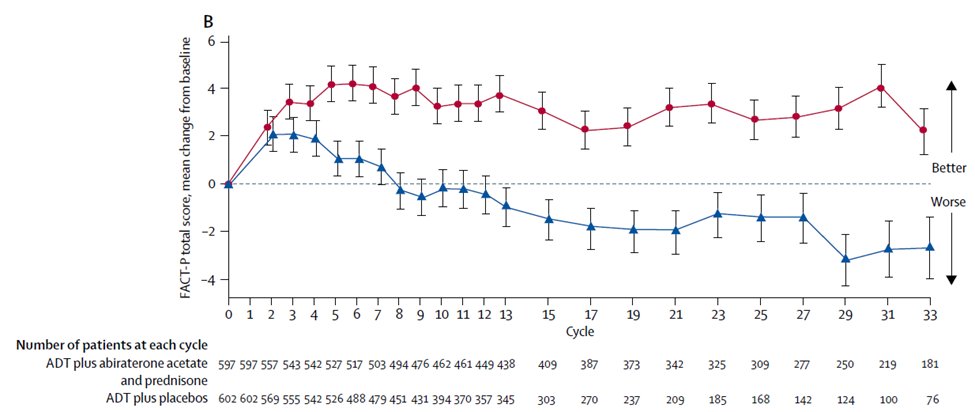
In TITAN, no difference in patient-reported outcomes was apparent between treatment with apalutamide and placebo arms using the FACT-P total score and the same was true in ARCHES for patients treated with enzalutamide. A longitudinal comparison of abiraterone and docetaxel suggested statistically significantly higher global quality of life scores for those patients receiving abiraterone.
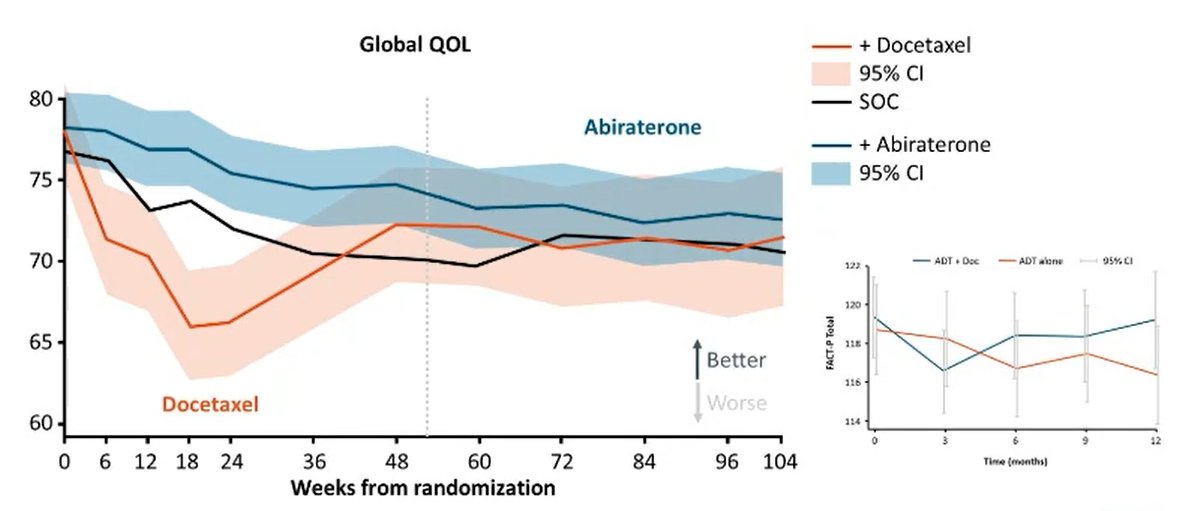
Given all these data, Dr. Morgans emphasized the importance of understanding the decision-making process for each patient. Notably, patients may consider a variety of other factors including cost, convenience, and impacts of activities of daily living.
She then concluded that treatment intensification with either docetaxel or an androgen receptor targeting agent is the standard of care for patients with mCSPC. The choice among these agents depends on quality of life and patient preferences in the context of shared decision-making.
Presented by: Alicia Morgans, MD, MPH GU Medical Oncologist, Dana Farber Cancer Institute, Boston Massachusetts
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


