(UroToday.com) In this late breaking abstract, Zhao and colleagues delved into the reporting of tobacco use in contemporary GU clinical trials and found that it is rarely collected or reported. Considering the impact of smoking on the development of GU malignancies, this is a deficiency that needs to be addressed.
He starts by reviewing the impact of tobacco use on genitourinary malignancies. Smoking or tobacco use is an established causal or contributory factor for nearly all genitourinary cancers. It also subsequently exerts a significant influence on treatment, quality of life, and survival outcomes (cancer-specific and overall survival). Therefore, as the authors note, in order to understand the influence smoking has on the outcomes of contemporary therapies, pertinent smoking-related data must be systematically collected and report. Unfortunately, as noted in a 2012 study of 155 actively accruing NCI Cooperative group trials, 29% assessed smoking at enrollment and only 4.5% assessed smoking at follow-up. As such, they sought to determine how often and how rigorously smoking status is collected and reported in publications of clinical trials in genitourinary cancers.
They conducted a systematic review (according to PRISMA guidelines) of high impact urology, general medicine, and oncology journals to identify manuscripts that reported the results of phase II-IV randomized clinical trials for the following GU malignancies: prostate, urothelial (bladder and UTUC) and kidney cancer. Manuscripts were screened in three phases by two independent reviewers; any conflict was resolved by a third reviewer with content expertise. Studies were included if they were published between May 2010-May 2020, reported results of a Phase II-IV randomized trial with a therapeutic intervention, and reported planned analysis of primary data. Pilot or Phase I studies, reports of unplanned secondary analyses, and studies with diagnostic/screening interventions were excluded. Their primary outcome of interest was whether a study collected and reported data on participant smoking status. Secondary outcomes included details about how smoking status was reported, whether the trial arms were balanced with respect to smoking status, and if smoking status was included in analysis.
Their initial search yielded 622 articles, of which 354 met inclusion criteria. Flowchart of study is seen below:
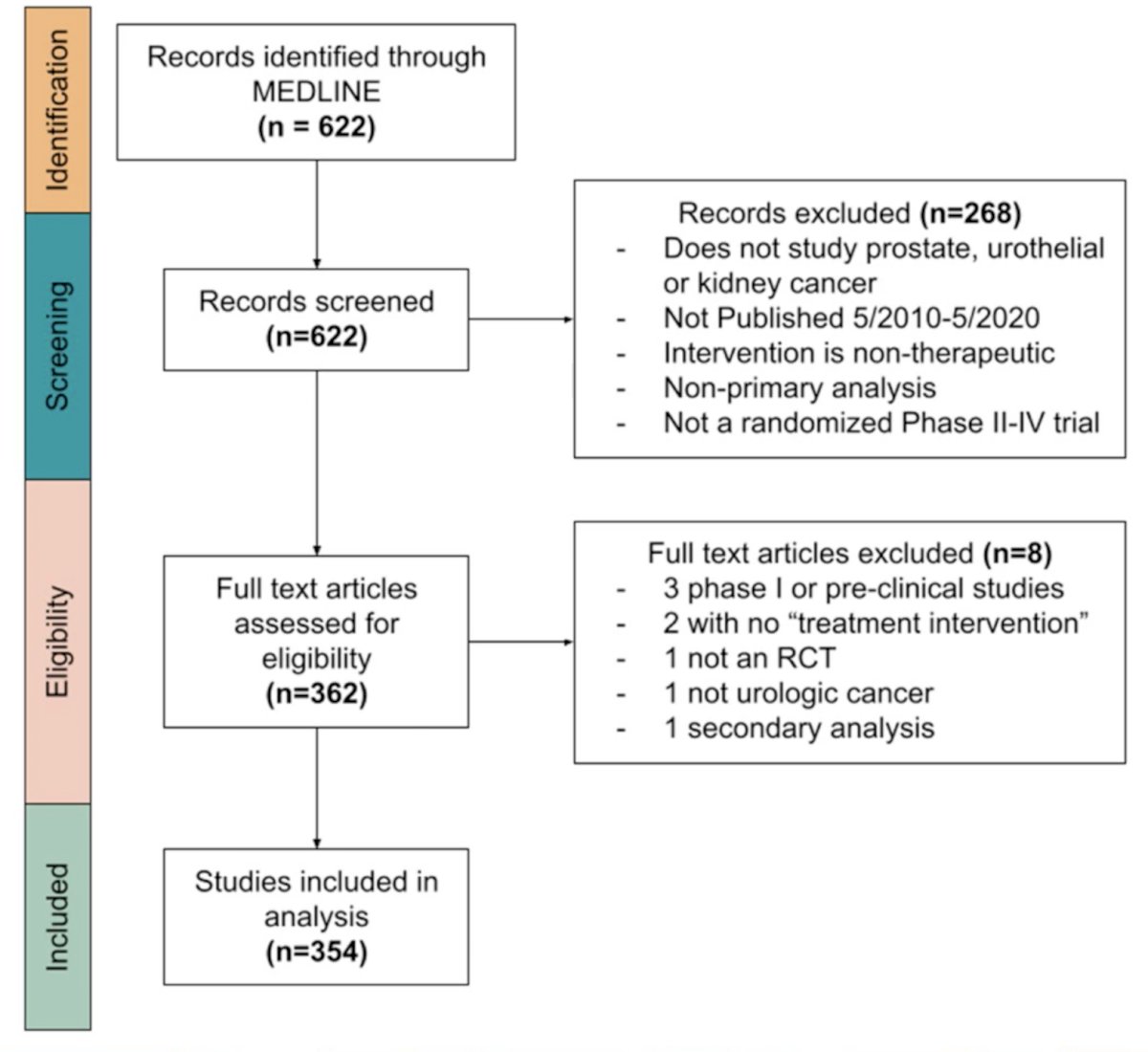
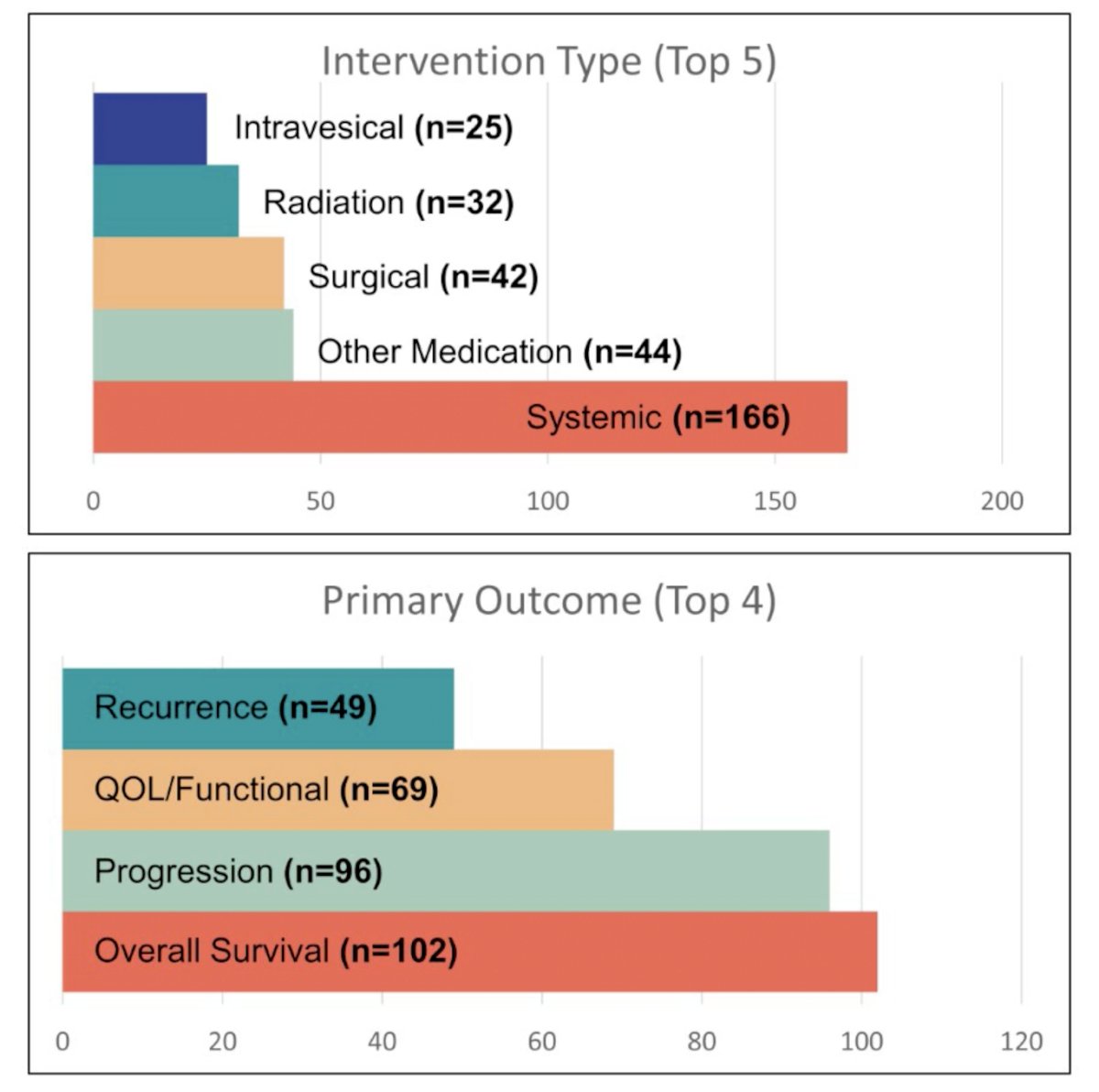
Outcomes of trials in prostate cancer (59.3%) and those studying systemic therapies (46.9%) were most common. Most were Phase III studies (69.8%) and included patients with either localized (33.9%) or metastatic (33.9%) disease.
- JCO, EU, Lancet Oncology, Journal of Urology and NEJM were 5 most common journals
Intervention type and primary outcomes are summarized below:
When looking at smoking status, the vast majority of included studies (N=324, 91.8%) did not report any details about trial participants’ smoking status. Of the 30 studies where it was included, 3 only reported in the supplement. When included, 96.3% of studies reported baseline status qualitatively (never, former, current) rather than quantitative (pack-years). No studies used a validated measurement instrument or reported change in participants’ smoking status over the study period. Smoking status was balanced in each arm in 22 studies.
None reported on non-cigarette tobacco use and none reported on smoking status changes during the study.
When included in final analysis (3 studies), smoking had a significant association with the primary outcome in two of three studies.
The authors appropriately conclude that clinical trial participants’ smoking status is rarely collected and reported in publications of genitourinary cancer trials. The absence of these data precludes further study of how smoking impacts outcomes and highlights an important deficiency in GU oncology clinical trial design. This deficiency should be remedied.
They finished by recommending the use of a validated and standardized instrument, such as the Cancer Patient Tobacco Use Questionnaire from the NCI. Also, smoking data should be integrated into EMR so data collection can be more seamless. Smoking cessation should become an important discussion point in any patient who is actively smoking.
Presented by: Calvin Zhao, BS, 4th year medical student, NYU Grossman School of Medicine
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


