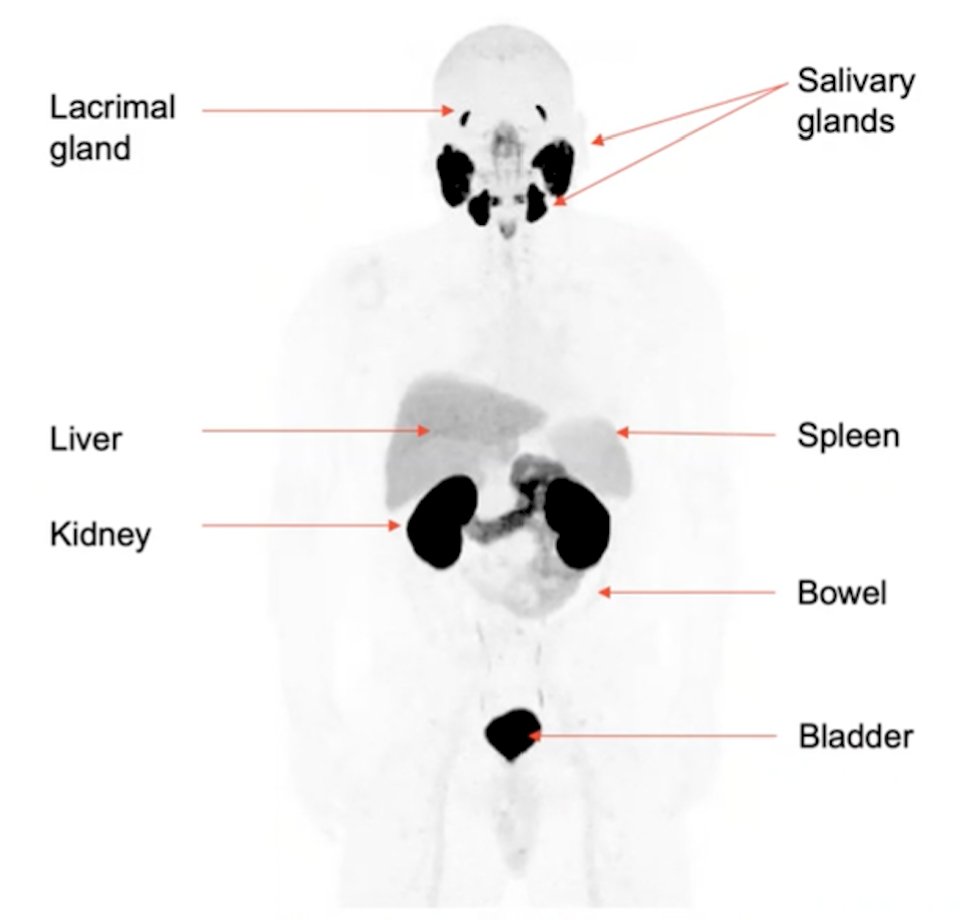
(UroToday.com) The American Urologic Association (AUA) annual meeting included a late-breaking abstract session with a presentation by Dr. Andrei Iagaru discussing the prospective evaluation of 18F-DCFPyL PET/CT in biochemically recurrent prostate cancer and the detection of extra-pelvic oligometastases. 18F-DCFPyl has been shown to have intense uptake in the bladder, kidneys, salivary glands, and lacrimal glands, with weak uptake in the liver, spleen, and bowel:
At the AUA session, Dr. Iagaru and colleagues presented the results of their study prospectively evaluated 18F-DCFPyL in a single-center study for detecting recurrent lesions in prostate cancer patients with biochemical recurrence.
This study prospectively enrolled 242 men (49-91 years old, mean ± SD: 69.6 ± 8.0) with biochemical recurrence (PSA median 2.0 ng/mL, range 0.12 to 698.4) after primary definitive treatment with prostatectomy (72%), radiotherapy (28%) or both (25%). The 18F-DCFPyL positive lesions compatible with prostate cancer were evaluated by two independent readers. Patients with possible extra-pelvic oligometastases (1-3 lesions with 18F-DCFPyL uptake) were identified. The maximum standardized uptake values (SUV max) of 18F-DCFPyL in these lesions were measured and used to categorize lesions into high uptake (SUV max = parotid gland SUV mean), moderate uptake (parotid gland SUV mean > SUV max = liver SUV mean) and mild uptake (liver SUV mean > SUV max = blood pool SUV mean). The time sequence between 18F-DCFPyL injection, CT, and PET in this study is as follows:

18F-DCFPyL PET/CT had an overall positivity rate of 81% (172 scans), which increased with higher PSA levels (ng/mL): 65% (PSA <0.5), 74% (0.5 <= PSA <1), 90% (1 <= PSA <2), 93% (2 <= PSA <5) and 96% (PSA >= 5), respectively. In the cohort who underwent prostatectomy, 18F-DCFPyL PET/CT had a higher positivity rate in patients with shorter PSA doubling time (PSADT) (90% in PSADT 0-3 months versus 61% in PSADT > 12 months, p < 0.01):

Higher positivity rate was also observed in prostatectomy patients with high Gleason score (97% in Gleason score 9-10 versus 82% in Gleason score 8 versus 73% in Gleason score 7 and 67% in Gleason score 6, p < 0.01). There was no difference of 18F-DCFPyL positivity rate observed in post-radiation patients with different PSADT or Gleason scores. There were 84 patients (43%) that had oligometastatic disease (1-3 lesions) including 67 patients with extra-pelvic lesions:

The impact on patient management after 18F-DCFPyL PET/CT scan is as follows:

As a clinical example, Dr. Iagaru presented the case of a 69-year old man with biochemical recurrence (PSA 0.4 ng/dL), with an 18F-DCFPyL PET showing several small bones and a pelvic lymph node. Focal 18F-DCFPyL uptake was seen in the right ischium, noted on axial PET, CT, and fused PET/CT:

Dr. Iagaru concluded his presentation of 18F-DCFPyL PET/CT among patients with biochemically recurrent prostate cancer highlighting that 18F-DCFPyL PET/CT holds great potential to be a “one-stop-shop” diagnostic tool in the work-up of biochemically recurrent prostate cancer to identify possible extra-pelvic oligometastases for locally targeted therapy.
Presented by: Andrei Iagaru, MD, Stanford University, Palo Alto, CA
Co-Authors: Hong Song, Judy Nguyen, Farshad Moradi, Carina Mari Aparici, Ben Franc, Guido Davidzon
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


