(UroToday.com) The American Urologic Association (AUA) annual meeting included a late-breaking abstract session with a presentation by Dr. Michel Pavic discussing results of the AB12003 trial assessing masitinib plus docetaxel as first-line treatment of metastatic castration-resistant prostate cancer (mCRPC). Masitinib is an oral, small molecule drug that targets mast cell and macrophage activity. These innate immune cells are critical components of the tumor microenvironment and are associated with prostate cancer progression. Previously, a small trial (AB07004) showed that masitinib plus docetaxel slowed progression in patients with mCRPC.
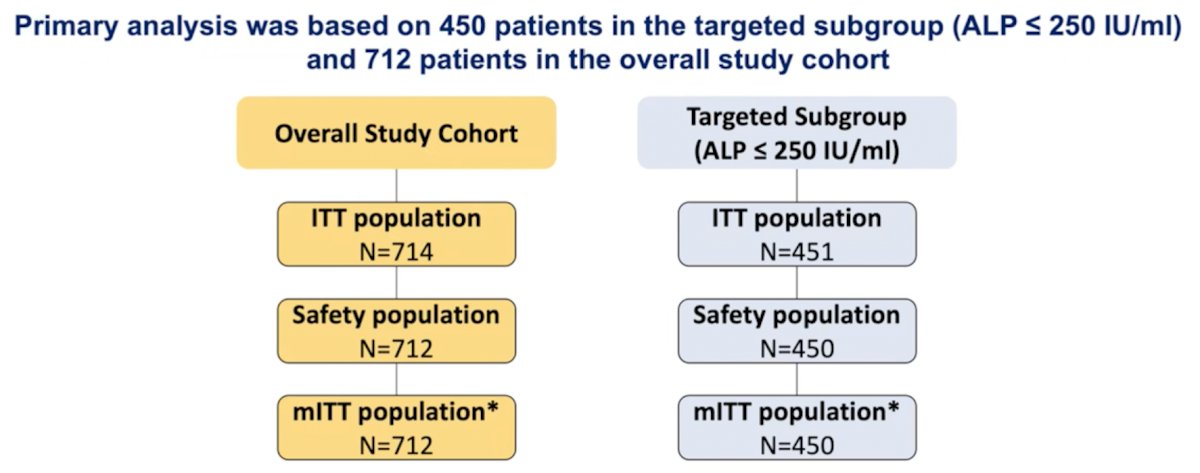
AB12003 was a prospective, placebo-controlled, double-blind, randomized, phase 3 trial, evaluating masitinib (6.0 mg/kg/d) in combination with docetaxel (IV 75 mg/m² plus prednisone for up to 10 cycles) as the first-line treatment of mCRPC. Eligible patients were chemo-naïve with confirmed mCRPC, who had progressed on previous abiraterone treatment or were indicated for docetaxel treatment and had a ECOG <=1. Primary analysis was performed on a pre-specified targeted subgroup, defined as patients with baseline alkaline phosphatase levels (ALP) <= 250 IU/ml, and on the overall population.
The primary endpoint was progression-free survival (PFS) (PCWG2 definition). The study was successful if improvement in median PFS relative to control reached a 3.9% level of significance for the target subgroup (alpha split with fallback procedure to conserve overall type-I error at 5% for the overall study cohort).
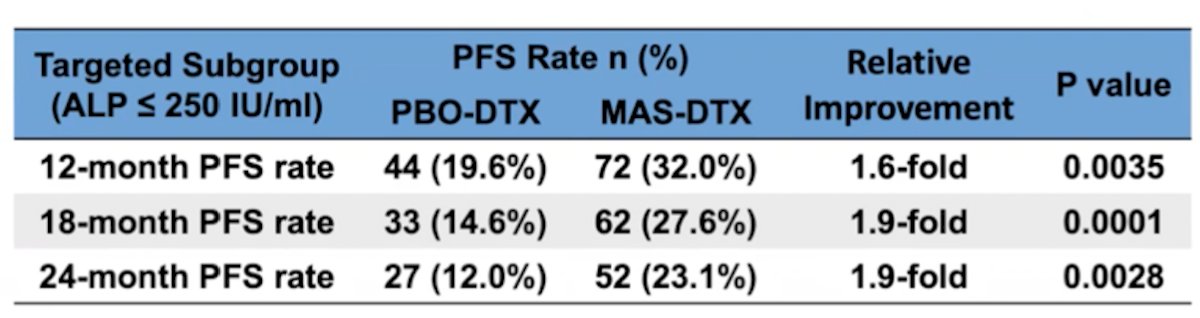
A total of 714 patients were enrolled (safety population n = 712). In the targeted (ALP <= 250) subgroup, masitinib-docetaxel (n=225) showed significant benefit over placebo-docetaxel (n = 225) with median PFS of 6.3 months (96.1% CI 5.6-7.6) versus 5.4 (96.1% CI 4.6-6.0) months; p=0.0272. The hazard ratio (HR) was 0.79 (96.1% 0.64-0.97), a significant 21% reduction in risk of progression for masitinib-docetaxel patients relative to control. Assessment of PFS rates was convergent with this primary outcome; 12, 18, and 24-month PFS rates showed significant 1.6-fold (p = 0.0035), 1.9-fold (p = 0.0001) and 1.9-fold (p = 0.0028) improvement, respectively, in favor of masitinib-docetaxel (i.e. 32.0%, 27.6% and 23.1% for masitinib-docetaxel vs 19.6%, 14.6% and 12.0% for placebo-docetaxel):
Dr. Pavic concluded his presentation of the AB12003 trial with the following concluding remarks:
- Masitinib (6.0 mg/kg/day) plus docetaxel confers a significant PFS benefit (21% reduced risk of progression) in mCRPC patients with ALP <= 250 IU/mL
- The masitinib-docetaxel safety profile was acceptable with respect to control
- The positive outcome of the AB12003 study provides further clinical evidence associating mast cells with the pathophysiology of mCRPC
- The combination of masitinib plus docetaxel may provide a new first-line treatment option for mCRPC patients with low metastatic involvement
Presented by: Michel Pavic, MD, PhD, Centre Hospitalier Universitaire de Sherbrooke (CHUS), Sherbrooke University, Sherbrooke, Quebec, Canada
Co-Authors: Olivier Hermine, Dominique Spaeth
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


