(UroToday.com) The 2023 American Urological Association (AUA) annual meeting held in Chicago, IL between April 28 and May 1st, 2023, was host to an invasive bladder cancer podium session. Dr. Ruiyang Xie presented his group’s work evaluating the tolerability and efficacy of BCG administration after recurrence in bladder-preserving therapy for muscle-invasive bladder cancer.
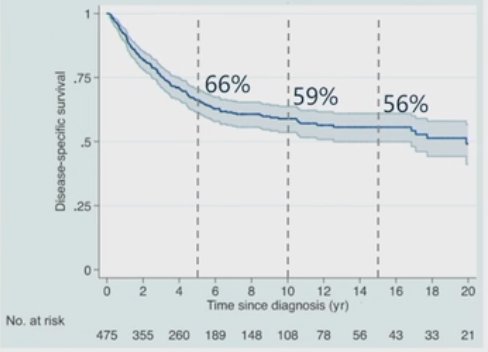
Trimodality therapy (TMT) has emerged as a standard of care treatment option for select patients with muscle-invasive bladder cancer. Numerous series have demonstrated the efficacy and safety of this approach, with the Mass General cohort study demonstrating 5-, 10-, and 15-year survival rates of 66%, 59%, and 56%, respectively.1
In this study, the authors sought to evaluate outcomes of patients with muscle invasive disease treated with TMT who subsequently experienced non-muscle invasive recurrences.
In this study, the authors enrolled patients with cT2 disease, all of whom underwent a maximal TURBT, and those with cT3-T4a disease who had a cystoscopic biopsy. All patients subsequently received gemcitabine + cisplatin. All patients subsequently underwent a maximal TURBT, following which patients with evidence of residual pT0, pT1, and pTa disease received concurrent chemo-radiotherapy. Subsequently, patients with intravesical NMIBC recurrences underwent a TURBT plus BCG.
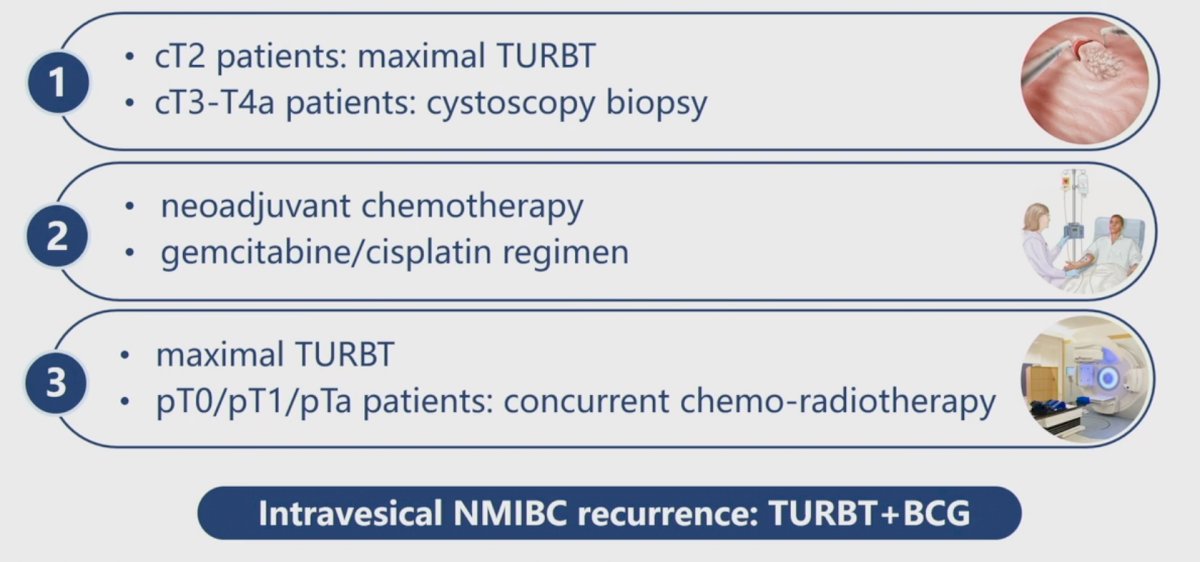
The BCG protocol was similar to the published SWOG BCG protocol, with the exception that patients did not receive the 6-month maintenance dose.

Between 2012 and 2021, 19 patients experienced NMIBC recurrence following TMT for MIBC. All 19 patients completed induction BCG, with 11/19 initiated on maintenance treatment. The most common adverse events observed were:
- Bladder irritation (90%)
- Hematuria (16%)
- Fever (5%)
- Arthritis (5%)
- Bladder tuberculosis (5%; 1 patient)
With regards to the efficacy outcomes, at a median follow-up of three years, the disease-free survival at 1 and 3 years was 71% and 65%, respectively. The corresponding OS rates were 89% and 81%, respectively.

How do these results compare to those for high-risk NMIBC patients from the EORTC cohort? As noted below, the disease-free survival for such patients was 70% at 1 year,2 similar to the observed rate of 71% in this cohort. As such, it appears that short-term oncologic outcomes for these patients are comparable to those observed in patients with NMIBC disease who had not received prior TMT for MIBC.

In summary, it appears that the tolerability and efficacy of BCG instillations for patients with NIMBC recurrence post-TMT is acceptable and similar to those observed with high-risk NMIBC patients from the EORTC cohort.
Presented by: Ruiyang Xie, MD, Urologist, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023References:
- Giacalone NJ, et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur Urol, 2017.
- Brausi M, et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol, 2014.


