(UroToday.com) The 2023 American Urological Association Annual meeting included a late breaking oncology abstract session. Dr. Andrea Necchi presented results from a clinical trials pipeline that assessed the biology and performance of pre- and post-pembrolizumab (Pembro) Vesical Imaging-Reporting and Data System (VI-RADS) to predict the pathological response in muscle-invasive urothelial bladder cancer (MIBC).
Dr. Necchi began with an overview of MIBC staging, noting the challenges associated with staging accuracy for both local tumor extent, as well as assessment of regional lymph nodes. He suggested that improvements in imaging techniques may address this issue, and have significant implications in staging strategies, assessing treatment response, identifying outlier responders, and recognizing patients at risk for early recurrence post-surgery. Since 2018, the introduction of the VI-RADS system has been studied in various settings, but he noted that there were no assessments evaluating the therapeutic response of MIBC to neoadjuvant chemotherapy (NAC) by using matched pre- and post-treatment MRIs. Therefore, the study authors sought to apply this imaging protocol within the PURE-01 study, which evaluated neoadjuvant pembrolizumab in MIBC patients.
The study schema was as follows:

Baseline characteristics of the trial cohort are noted below:

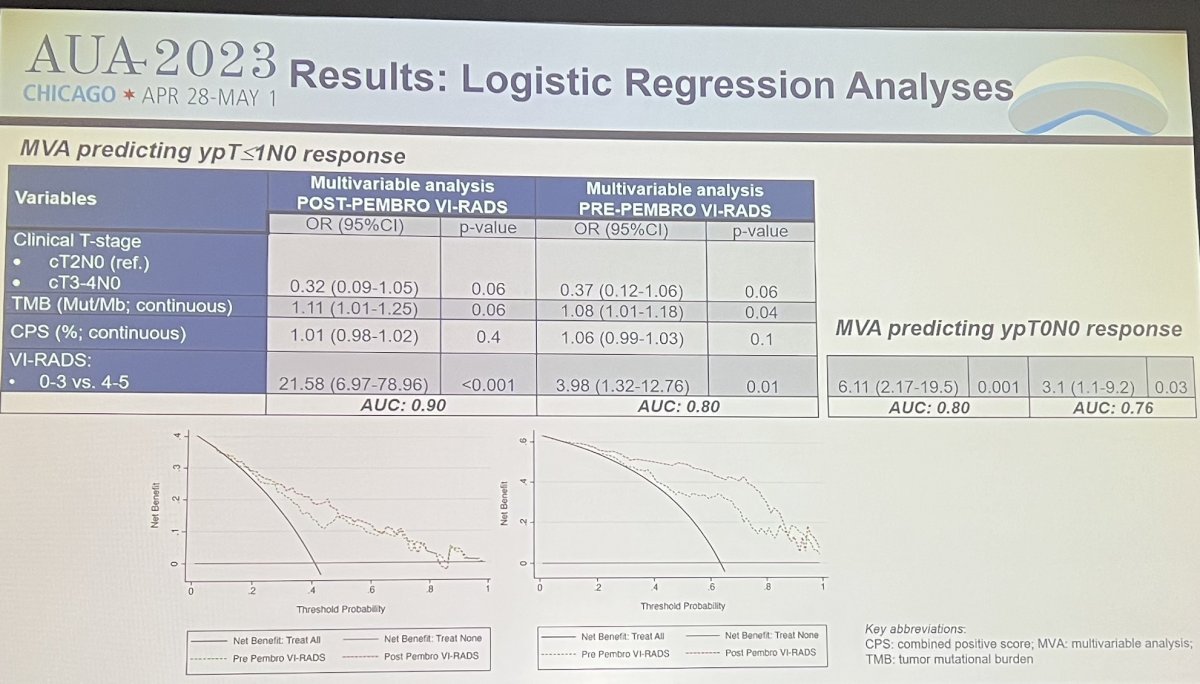
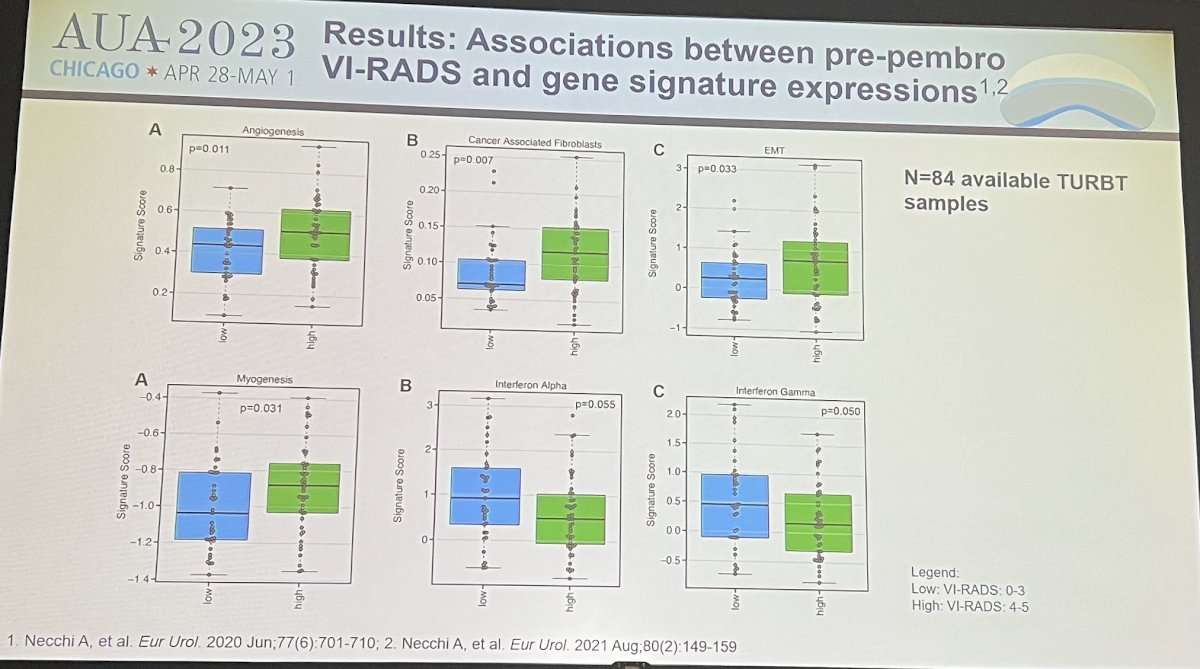
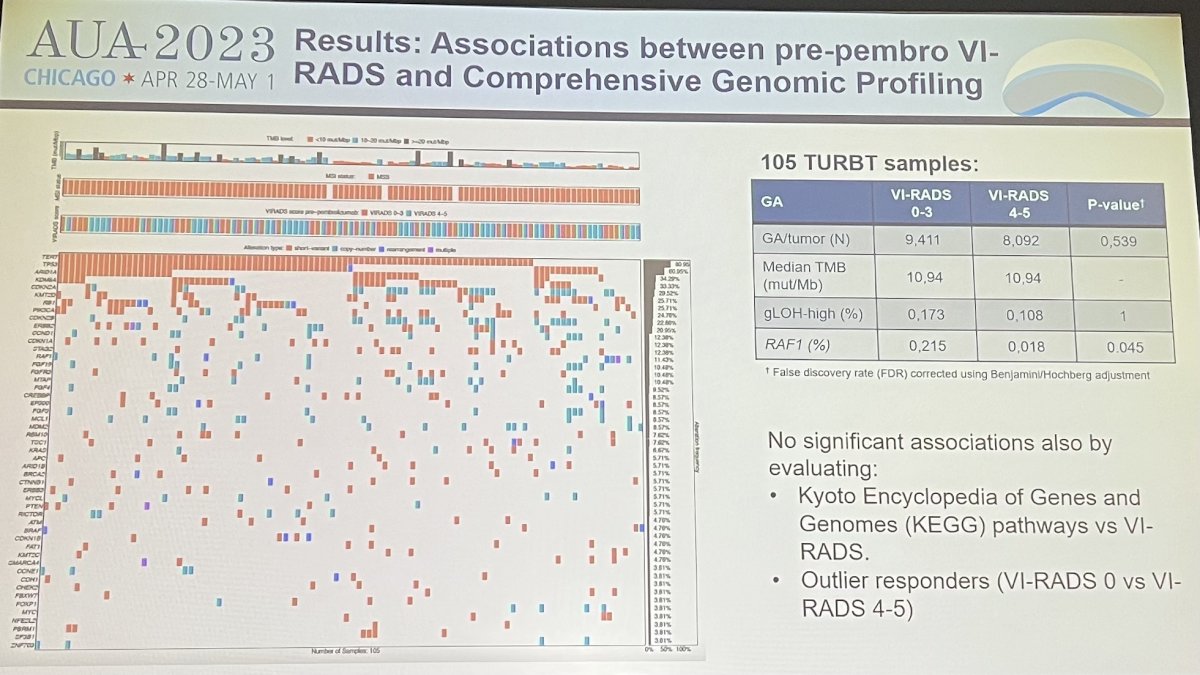
Dr. Necchi next reviewed results of the study. Before pembrolizumab, 21 pts (18.3%) had no evidence of measurable disease (VI-RADS=0), 37 (32.2%) had a VI-RADS score between 1-3, and 57 (49.5%) had a VI-RADS score between 4-5. Overall, 20 pts (17.4%) were downstaged on MRI from VI-RADS 4-5 to VIRADS 0-3 after pembrolizumab treatment. Before pembrolizumab treatment, VIRADS scores between 4-5 were associated with higher angiogenesis and epithelial-mesenchymal transition (EMT) activity as compared to lower pre-pembro VI-RADS score between 0-3 (p=0.011 and 0.033). Before and after pembrolizumab therapy, VI-RADS 0-3 scores were the only significant covariates against the primary endpoint of pT1 disease rate on multivariable analyses. The strongest effect was noted in post-pembro VI-RADS scores of 0-3 against the pT1 response endorpoint (OR: 23.4, 95%CI: 7-95.3, p<0.0001, AUC 0.90).
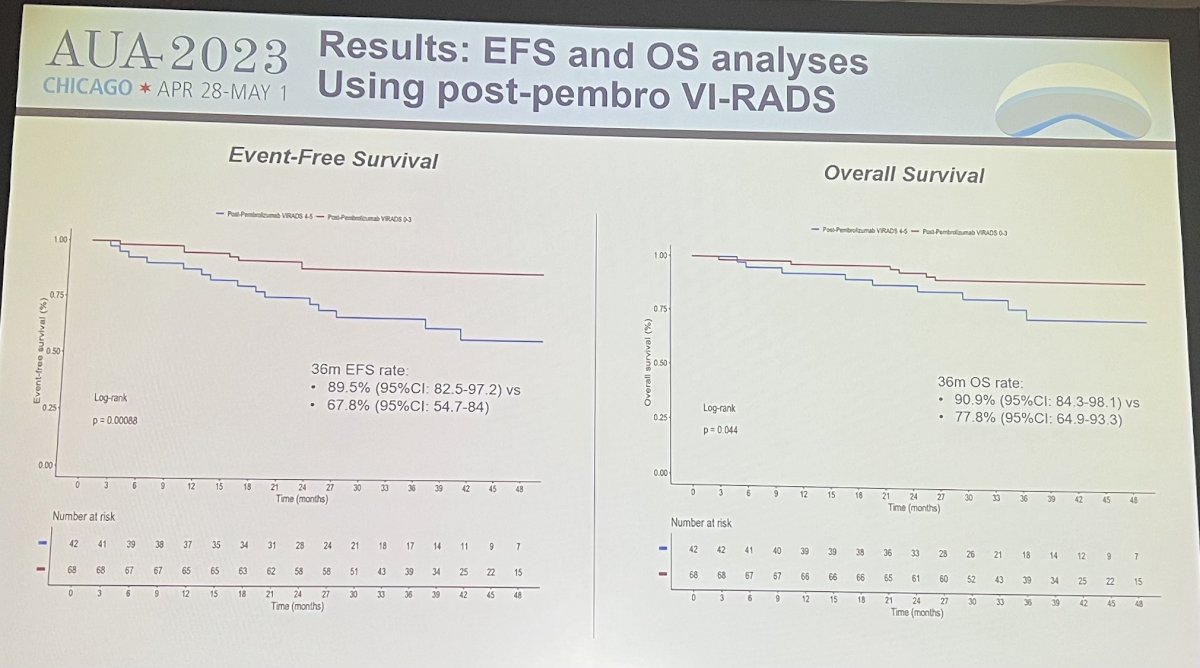
VI-RADS score of 4-5 after pembrolizumab were characterized by higher stromal signature scores as opposed to VI-RADS of 0-3 after pembrolizumab. When post-pembrolizumab VI-RADS scores were tested in evaluable pts from the combined NureCombo+SURE-01 cohort (N=17; 34 total mpMRI scans), the majority, 90%, revealed pT1 stage at the time of surgery.
Dr. Necchi concluded his presentation by noting the following:
- MRI with VI-RADS assessment to evaluate the response to neoadjuvant pembrolizumab in MIBC revealed a way for noninvasive prediction of the pathological response and outcome prior to surgery, potentially allowing for better selection of patients for bladder-sparing strategies
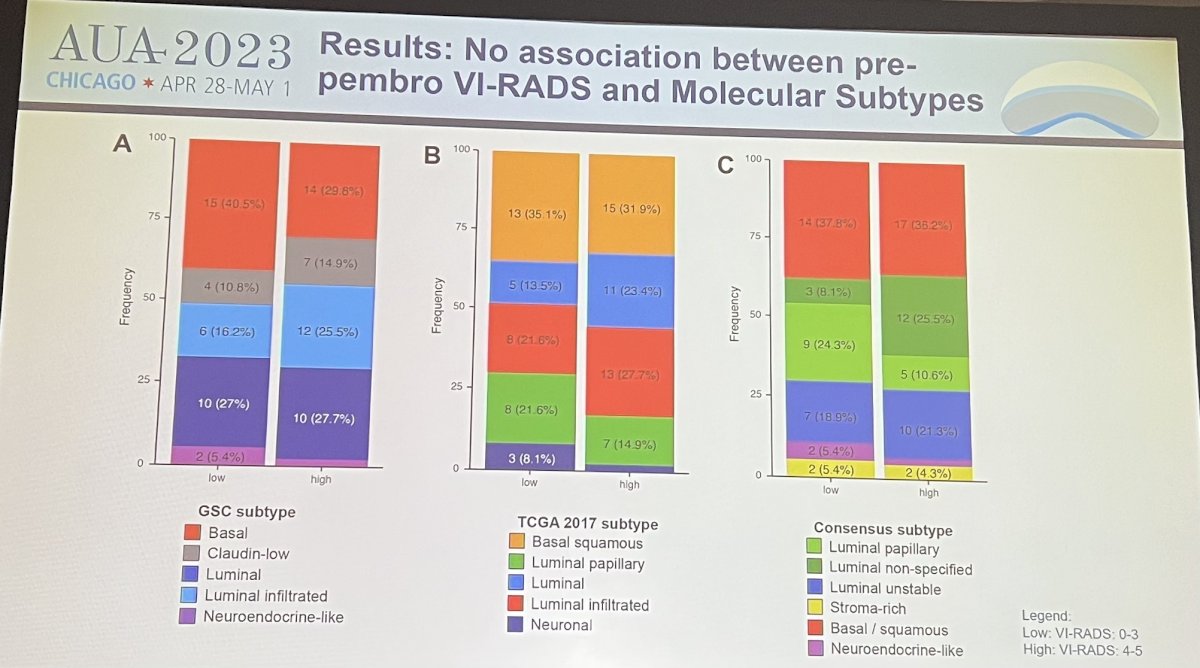
- VI-RADS scores were correlated with the probability of residual MIBC after TURBT, as well as biological features associated with response to pembrolizumab
- Based on this, MRI should be offered in clinical practice to improve assessments of response to treatment in the neoadjuvant IO setting
Presented by: Andrea Necchi, MD, Fondazione IRCCS Istituto Nazionale dei Tumori, Medical Oncologist, San Raffaele Hospital and Scientific Institute, Milan, Italy
Written by: Ruchika Talwar, MD, Urologic Oncology Fellow, Vanderbilt University Medical Center, during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:
- Tokes A, et al. Am J Roonigenol 2005
- Vargas HA, et al. Prospective evaluation of MRI, ¹¹C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol 2012
- Panebianco V, et al. Negative Multiparametric Magnetic Resonance Imaging for Prostate Cancer: What's Next?. Eur Urol 2018
- Del Giudice F, et al. Prospective Assessment of Vesical Imaging Reporting and Data System (VI-RADS) and Its Clinical Impact on the Management of High-risk Non-muscle-invasive Bladder Cancer Patients Candidate for Repeated Transurethral Resection. Eur Urol 2020
- Pocoraro M, et al. Am J Roonigenol 2020
Full Data Analysis of the Biology and Performance of Pre- and Post-Pembrolizumab VI-RADS for Predicting Pathological Response in MIBC: Insights from a Comprehensive Clinical Trials Pipeline - Andrea Necchi


