(UroToday.com) The 2023 AUA annual meeting included the Bladder Cancer Forum, featuring a debate discussing whether we should perform a radical cystectomy or intravesical therapy for very high risk non-muscle invasive bladder cancer (NMIBC). Dr. Sarah Psutka started the session by presenting a case of a 74 year old female referred for evaluation of intermittent gross hematuria for 3 months, which was attributed to kidney stones and a UTI. Additional associated symptoms included nocturia every 2 hours and dysuria, with no improvement on antibiotics. Her primary care physician ordered a CT scan showing multiple large bladder masses, no lymphadenopathy, and no distant metastasis, and no hydronephrosis:
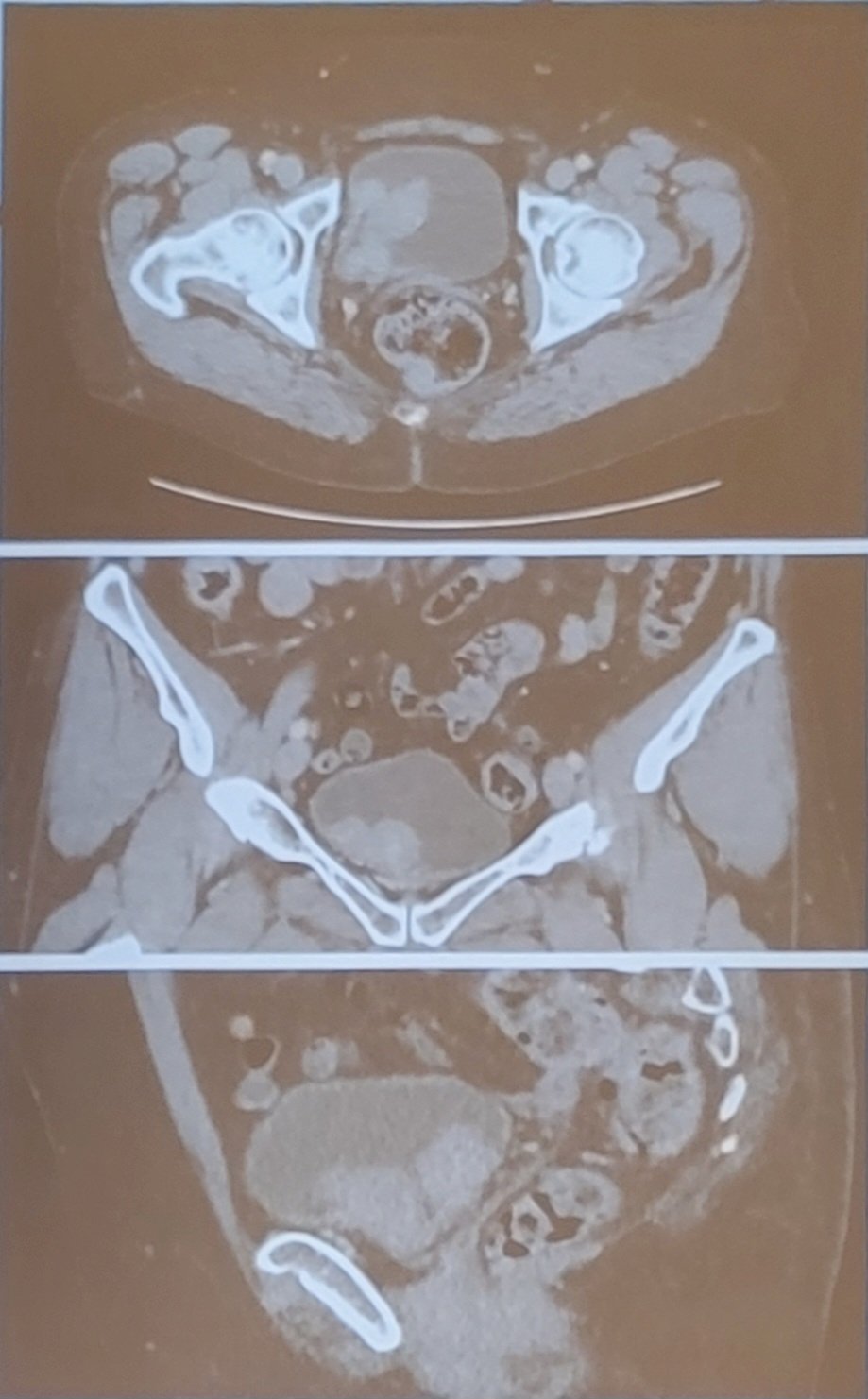
The patient’s medical history was consistent with overactive bladder, fibroids, hypertension, diabetes, and chronic kidney disease; surgical history included total abdominal hysterectomy and an appendectomy. Her social history included 0.5-1 pack per day smoking history for 42 years and she worked as a hair dresser. Her creatinine was 1.1 and her hepatic function was normal. The patient then had a TURBT, which demonstrated a normal examination under anesthesia with no palpable masses and a mobile bladder. There were numerous large 3-6 cm highly vascular papillary tumors on the right bladder wall and base, obscuring but not involving the ureteral orifice, with a visually complete resection. In total >10 cm in tumor was resected and pathology showed high grade T1 urothelial carcinoma with muscularis propria present and negative for tumor and no CIS. Four weeks later the patient had a re-TURBT that showed significant eschar across the resection bed surrounded with erythema, a small amount of recurrent versus persistent papillary tumor at the bladder neck, with a visually complete resection. The pathology report from the re-TURBT was high grade Ta and high grade T1 urothelial carcinoma, focally suggestive of CIS, with muscularis propria present and uninvolved by tumor. Dr. Psutka then polled the audience asking “What treatment would you recommend for this patient?”
- 12%: Induction intravesical BCG followed by maintenance BCG for 1 year
- 48%: Induction intravesical BCG followed by maintenance BCG for 3 years
- 4%: Induction intravesical gemcitabine/docetaxel (6 weeks)
- 36%: Radical cystectomy
Dr. Fred Witjes then took the position of immediate radical cystectomy for this patient. Why is pT1 high grade disease so risky? Primarily, some tumors are more than NMIBC secondary to understaging. Although we are getting better at TURBT, likely secondary to better equipment, around 10% of T1 tumors are still understaged. Dr. Witjes notes that perhaps MRI may help in appropriately staging these patients. A recent systematic review and meta-analysis from Cornellssen et al.1 assessed 20 studies, including 1,724 patients for predicting local staging. The pooled analysis showed a sensitivity of 0.92 and specificity of 0.88 for differentiating stages <=T1 and >=T2. Important factors included patient number (ie. experience), magnetic field strength (1.5 vs 3 Tesla), image slice thickness, and VI-RADS cutoff score. As follows are representative imagines of T1 and >=T2 lesions:
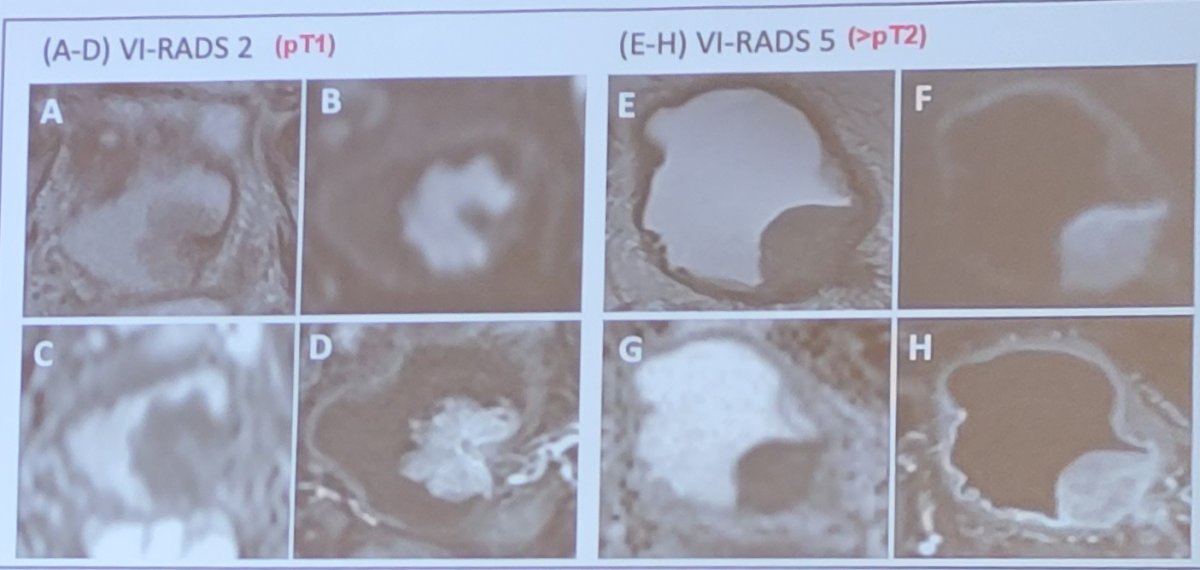
Dr. Witjes emphasized that there is progression in high risk NMIBC. Population level studies suggest that this rate of progression may be as high as 23% at 5 years. A 2016 update of the EORTC nomogram2 assessed 1,812 patients treated with 1-3 years of BCG with a median follow-up of 7.4 years. The end point for this study was early and late recurrences, progression and death. Significant factors for recurrence included prior recurrence rate and number of tumors and for progression and death included stage and grade. In particular, T1 high grade tumors (not even very high risk) patients do poorly with 1-5 year progression rates of 11.4-19.8%.
More concerning is that in the case of progression for these patients that were NMIBC but become muscle invasive disease, cancer specific mortality after progression is as high as 60%-65% based on several studies. Dr. Witjes cautions that we need to beware of high risk (T1) NMIBC:
- Around 10% are understaged and are actually >=T2
- At least 20% will show progression to muscle invasive disease and >=50% will die of bladder cancer within 3 years
- Thus, the window of opportunity is very limited for these patients
To quote moderator of this session Dr. Ashish Kamat, T1 high grade bladder cancer has the same disease specific survival as cT3b, Gleason 5+5, 12/12 cores positive prostate cancer with a PSA of 75 ng/mL. However, the case is even worse for very high risk patients, particularly T1 high grade with CIS or multiple T1 tumors. Among these very high risk patients, females have a worse prognosis, thus he notes that for this patient she is at an increased risk of recurrence for the next 20 years, particularly with the re-TURBT still positive for T1 high grade and CIS. Furthermore, the bladder neck involvement is a nasty location for intravesical therapy, similar to the prostatic urethra. It may be even worse if we knew the T1 sub-classification and lymphovascular invasion. In a 2015 study by Martin-Doyle,3 among 15,215 patients, the most important risk fact for progression (HR 3.34) and cancer specific mortality (HR 2.02) was T1b/c disease. Additional factors associated with worse outcomes included lymphovascular invasion, CIS, non-BCG intervention, size >3 cm and older age.
For very high risk NMIBC, the guidelines state the following:
- AUA:
- High-risk and persistent or recurrent Ta or CIS disease after induction BCG a second course of BCG (Moderate Recommendation; Evidence Strength Grade C)
- High-risk and fit for surgery with persistent high-grade T1 disease on repeat resection, or T1 tumors associated with CIS, LVI, or variant histology offer initial radical cystectomy (Moderate Recommendation; Evidence Strength Grade C)
- High risk and persistent/recurrent disease within 1 year after 2 cycles of BCG or BCR maintenance offer radical cystectomy (Moderate Recommendation; Evidence Strength Grade C)
- EAU: Consider cystectomy or offer intravesical full dose BCG instillations for one to three years for those who refuse or are unfit for radical cystectomy (Recommendation: Strong)
Dr. Witjes concluded his presentation with his own personal recommendations:
- Rather be over treated and alive as compared to undertreated and dead
- In this 74 year old ECOG 0 lady – recommend a radical cystectomy pelvic lymph node dissection, urethral frozen section and urinary diversion
Dr. Seth Lerner then discussed intravesical therapy as the optimal therapy for this patient. Dr. Lerner notes that the AUA/SUO NMIBC guideline was amended in 2020, emphasizing high risk (EAU very high risk based on tumor volume), and the importance of re-resection to verify complete resection and rule out muscularis propria invasion. Furthermore, in these high risk patients, there is no role for a single dose of perioperative intravesical therapy. Similar to Dr. Witjes, Dr. Lerner highlighted the importance of T1 sub-staging, discussing the 2012 article by Van Rhijn et al.4 Among 134 patients with T1 as a first diagnosis, the muscularis mucosae-vascular plexus was not present at the invasion front in 50 patients (37%). Thus, T1 substage was as follows: 40 T1m (single focus <= 0.5 mm on one high powered field) and 94 T1e (larger or multiple areas); 81 T1a, 18 T1b, and 35 T1c. In multivariable analyses, substage (T1m/T1e) was significant for progression (p=0.001) and DSS (p=0.032), whereas substage according to T1a/T1b/T1c was not significant. Dr. Lerner advocates that pathologists should report depth of the extent of lamina propria invasion in TURBT specimens, with several methods being proposed:
- Micrometric (3 methods)
- Histoanatomic
- Head-to-head prospective comparisons are needed
Additionally, the invasive component should be assessed by depth and if not possible then the greatest dimension. En bloc resection may improve T1 staging and substaging.
According to the AUA/EAU guidelines, a 6 week induction course of BCG therapy for high risk tumors is acceptable. There should be consideration of early cystectomy for T1 high grade tumors with additional risk factors (CIS, LVI, variant histology, persistent T1 high grade on repeat TURBT, multiple or large T1 high grade tumors):
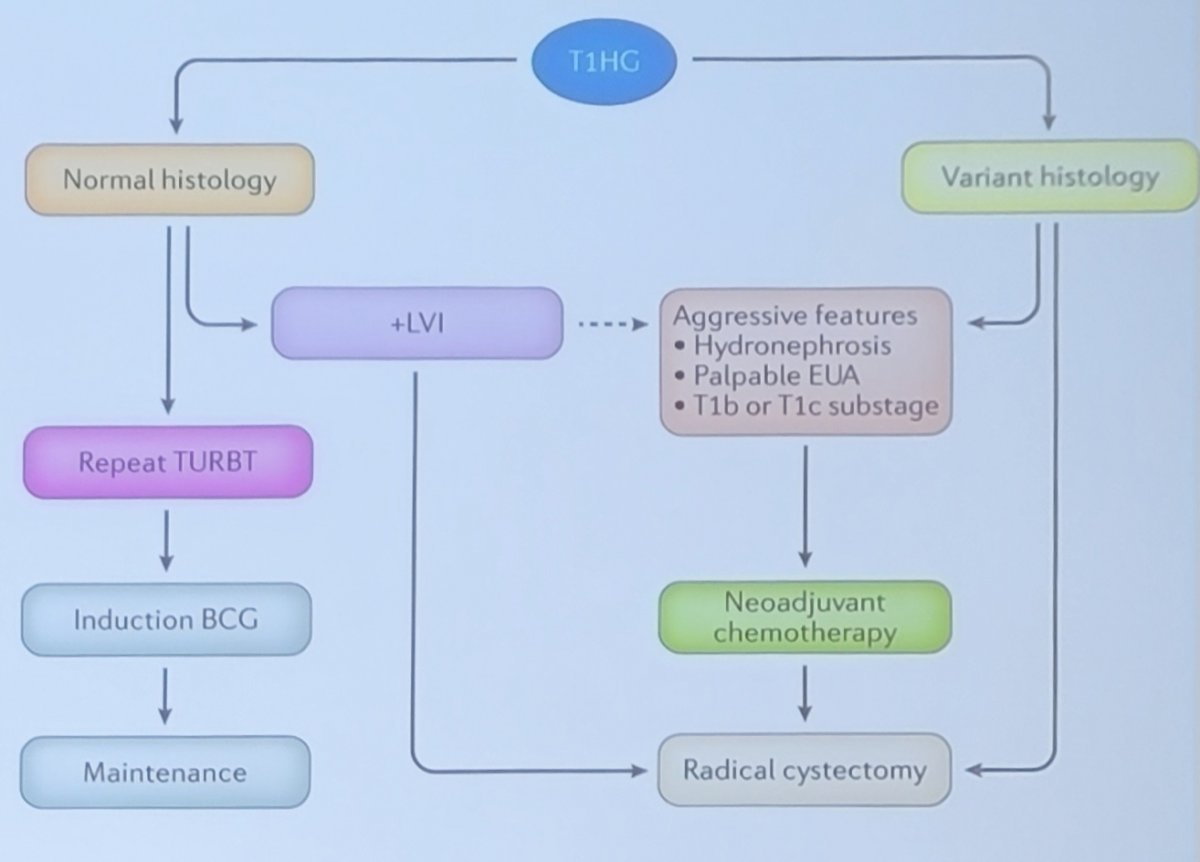
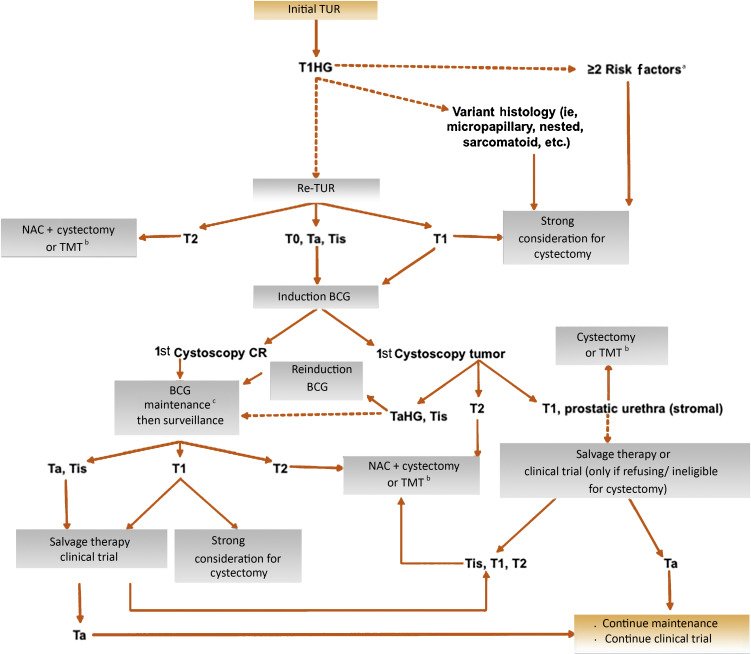
In 2018, Klaassen et al.5 provided treatment strategies for newly diagnosed T1 high-grade bladder urothelial carcinoma concluding that longer life expectancy, extensive CIS or variant histology, multifocal tumor, and residual T1 high grade disease at re-TUR should undergo immediate radical cystectomy, while patients without these features could reasonably be offered BCG with early radical cystectomy reserved for those with recurrent high grade disease despite adequate BCG. The authors proposed the following algorithm for treatment of T1 high grade bladder cancer:
Among contemporary studies assessing outcomes of T1 high grade disease managed conservatively with BCG, progression rates range from 6-31% and disease specific survival rates were 81-97%.5 Dr. Lerner also touched on the BRAVO trial,6 which attempted to randomize patients to radical cystectomy versus intravesical BCG for high-risk NIMBC. This trial was a two-arm, prospective multicenter randomized study to determine the feasibility in BCG-naive patients, in which patients had new high-risk NMIBC suitable for both treatment arms. There were 407 patients screened, of which 185 were approached for the trial, and consent was obtained from 51 (27.6%) patients. In the BCG arm, 23/25 (92.0%) patients received BCG, of which four had NMIBC after induction, three had NMIBC at 4 months, and four received radical cystectomy. In the radical cystectomy arm, 20 (80.0%) participants received cystectomy, including five (25.0%) with no tumor, 13 (65.0%) with high-risk NMIBC, and two (10.0%) with muscle invasion in their specimen.
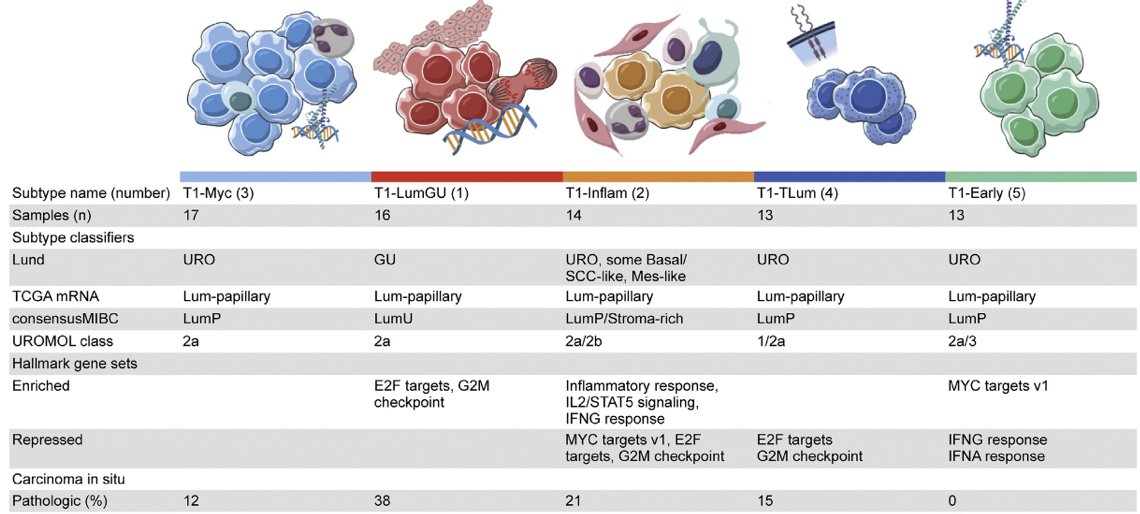
Robertson et al.7 not only did T1 subtyping, but performed transcriptome profiling and unsupervised clustering, identifying five consensus subtypes of T1 tumors treated with repeat transurethral resection and induction and maintenance BCG. The findings were as follows:
- T1-LumGU subtype: associated with carcinoma in situ, had high E2F1 and EZH2 expression, and was enriched in E2F target and G2M checkpoint hallmarks
- T1-Inflam subtype: was inflamed and infiltrated with immune cells
- T1-TLum subtype: had the highest median luminal papillary score and FGFR3 expression, no recurrence events, and the fewest copy number gains
- T1-Myc and T1-Early subtypes: had the most recurrences (14/30 within 24 months) and the highest median MYC expression
- T1-Early subtype: had five (38%) recurrences within the first 6 months of BCG, and repressed IFN-α and IFN-γ hallmarks and inflammation
Finally, Dr. Lerner highlighted the proposed treatment algorithm based on genomic characteristics predicting outcome in high-grade T1 bladder cancer:8

Dr. Lerner concluded his presentation with the following summary statements:
- This patient has high risk (AUA)/very high risk (EAU) T1 high grade disease based on large volume
- But, complete resection (2nd TURBT), no CIS, LVI or variant histology and age 74 with comorbidities, warrants induction BCG and careful assessment of response at 3 months
- Risk with upfront cystectomy: pT0N0 and therefore over treatment, 1-3% mortality, 15-25% major complication risk, diminished quality of life risk
- The future is genomic risk stratification
Dr. Psutka then re-polled the audience asking “What treatment would you recommend for this patient?”
- 4%: Induction intravesical BCG followed by maintenance BCG for 1 year
- 54%: Induction intravesical BCG followed by maintenance BCG for 3 years
- 4%: Induction intravesical gemcitabine/docetaxel (6 weeks)
- 38%: Radical cystectomy
Moderator: Sarah P. Psutka, MD, University of Washington, Seattle, WA
Debater: Seth P. Lerner, MD, FACS, Baylor School of Medicine, Houston, TX
Debater: J. Alfred Witjes, MD, PhD, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:
- Cornellssen SWE, Veenboer PW, Wessels FJ, et al. Diagnostic accuracy of multiparametric MRI for local staging of bladder cancer: A systematic review and meta-analysis. Urology. 2020 Nov;145:22-29.
- Cambier S, Sylvester RJ, Collete L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of Maintenance Bacillus Calmette-Guerin. Eur Urol 2016 Jan;69(1):60-69.
- Martin-Doyle W, Leow JJ, Orsola A, et al. Improving Selection Criteria for Early Cystectomy in High-grade T1 Bladder Cancer: A Meta-Analysis of 15,215 patients. J Clin Oncol. 2015 Feb 20;33(6):643-650.
- Van Rhijn BWG, van der Kwast TH, Alkhateeb SS, et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol 2012;61:378-384.
- Klaassen Z, Kamat AM, Kassouf W, et al. Treatment strategy for newly diagnosed T1 high-grade bladder urothelial carcinoma: New insights and updated recommendations. Eur Urol 2018 Nov;74(5):597-608.
- Catto JWF, Gordon K, Collinson M, et al. Radical Cystectomy Against Intravesical BCG for High-Risk High-Grade Nonmuscle Invasive Bladder Cancer: Results from the Randomized Controlled BRAVO-Feasibility Study. J Clin Oncol. 2021 Jan 20;39(3):202-214.
- Robertson AG, Groeneveld CS, Jordan B, et al. Identification of Differential Tumor Subtypes of T1 Bladder Cancer. Eur Urol. 2020 Oct;78(4):533-537.
- Bellmunt J, Kim J, Reardon B, et al. Genomic Predictors of Good Outcome, Recurrence, or Progression in High-Grade T1 Non-Muscle-Invasive Bladder Cancer. Cancer Res. 2020 Oct 15;80(20):4476-4486.


