(UroToday.com) Kidney cancer has seen an increasing incidence globally. Often, it is perceived at the localized stage. Currently, the gold standard modality for kidney cancer is surgical resection of renal cell carcinoma. With its expanding presence and risk for recurrence, it is important to thoroughly understand the prognosis of kidney cancer and develop a management plan that is specific to each patient. The existing prognostic scores have a limited ability to predict accurately, which poses a challenge for urologists to create effective postoperative care strategies and elect patients who would greatly benefit from adjuvant therapy. The need to recognize patients compatible with light surveillance or adjuvant therapy calls for a better prediction system. Dr. Gaelle Margue and her team sought to develop a machine learning model utilized for the purposes of individualized prediction of recurrence risk after surgery (5-year Disease Free Survival after surgery). She delivered a fascinating presentation about their findings at the American Urological Association (AUA) Annual Meeting.
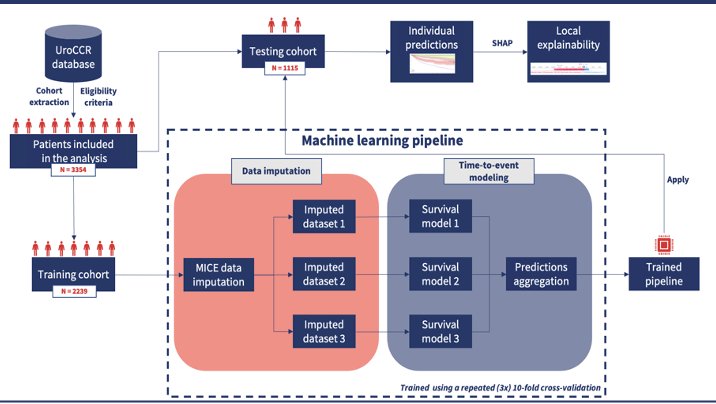
Figure 1: This figure depicts the workflow of this study: patient recruitment, training, and testing cohort, etc.
As seen in Figure 1, Dr. Margue and her colleagues recruited a cohort of patients from the French research network on kidney cancer database (NCT03293563), consisting of 38 hospitals. These patients underwent surgery (radical or partial nephrectomy) for localized advanced renal cell carcinoma (pT1-T4, N0, M0) between May 2000 and January 2020. Exclusion criteria included patients with benign lesions, genetic cancer, a concomitant malignant disease of inflammatory disease. Demographic, clinical, biological, radiological, and histologic data was recorded for each individual. Patients were randomly chosen to be a part of either the training or testing groups with a 67/33 ratio.
A total of 3354 patients were included to be a part of this study: 2239 in the training cohort and 1115 in the testing cohort. As seen in Figure 2, patient cohort had tumors with average size of 4 centimeters. 71% of patients had ccRCC, 3% presented with locoregional relapse and 7% experienced metastatic progression. Specific characteristics of each patient were extracted and used to train the algorithm. Multiple machine learning models were trained according to the training cohort and parameters of each were enhanced with repeated cross-validation methods. The optimization metric employed was the C-index measured at the 5-year mark. The models’ predictive performance was assessed using the testing cohort as well as C-index and AUC. Specifically, an integrated AUC was applied to quantify the discriminative capabilities of the model at each time point.
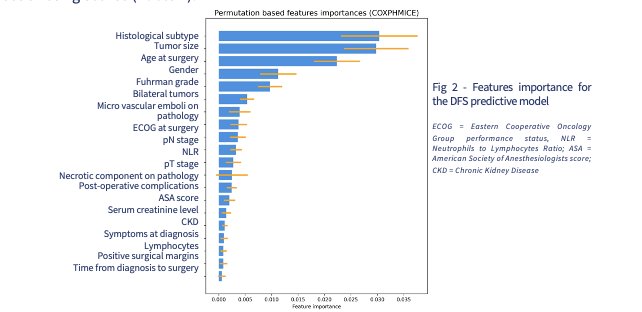
Figure 2: This figure displays the importance of each patient feature to the predictive model. Examples include tumor size, age, gender, etc.
Interestingly, Dr. Marque and colleagues found that their algorithm yielded the best results when using a Cox PH model with 18 variables. The most accurate method for predicting DFS was a MICE+Cox proportional hazards model that incorporated 24 features. This was demonstrated in Figure 3, where the model achieved an iAUC (0.5, 5 years) of 0.81 [IC95% 0.76 – 0.85]. In comparison, the UISS and SSIGN scores demonstrated C-index values of 0.61 and 0.72, respectively. Though the Leibovich scoring showed comparable AUC scores, Dr. Marque emphasizes that it is unable to provide a risk score with any incomplete data (Table 1). She notes that this novel algorithm can generate a risk score even with missing patient data. Moreover, Figure 4 presents SHAP values, which helped elucidate the individual contribution of each feature towards the patient-specific prediction of recurrence at 5 years.
Table 1: Predictive performances of alternative existing scores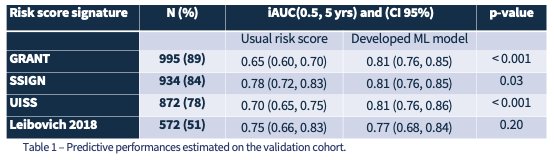

Figure 4: This figure displays an individual’s risk of relapse which can either increase or decrease the risk score as SHAP values.
Dr. Marque and her team concluded that applying machine learning to data from patients with localized kidney cancer who underwent surgery appears to yield more accurate individual predictions of disease-free survival when compared to conventional scoring methods. She highlighted its clinical implications, specifically in its ability to treat, inform, and advise patients.
Presented by: Gaelle Marque, MD, Bordeaux University Hospital, @gaellemargue on TwitterWritten by: Candices Tran, B.S., University of California, Irvine, @candicesmtran on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023


