(UroToday.com) The 2023 AUA annual meeting included an advanced prostate cancer session, featuring a presentation by Dr. Fred Saad discussing the PSA analysis from PROpel, the phase 3 trial of abiraterone + olaparib versus abiraterone + placebo as first-line treatment of mCRPC. PROpel met its primary endpoint of a significant radiographic progression-free survival (rPFS) benefit with abiraterone and olaparib vs abiraterone and placebo in the first treatment of patients with mCRPC enrolled irrespective of homologous recombination repair gene mutation (HRRm) status (HR 0.66, 95% CI 0.54–0.81).1
Exploratory analyses of rPFS favored abiraterone and olaparib vs abiraterone and placebo for patients with and without HRRm and/or a BRCA mutation. Additionally, a trend toward overall survival benefit with abiraterone and olaparib was observed. Furthermore, PSA is known to be regulated by the androgen receptor. At the 2023 AUA annual meeting, Dr. Saad and colleagues reported the results of a post hoc exploratory analyses of PSA response and time to PSA progression at a second data cut-off (March 14, 2022) for patients with and without HRR and/or BRCA mutations.
PROpel was a phase 3 double-blind trial. Patients were randomized 1:1 to receive olaparib (300 mg twice daily [bid]) or placebo, and abiraterone (1000 mg once daily) + prednisone/prednisolone (5 mg bid) until disease progression, unacceptable toxicity, or withdrawal of consent:
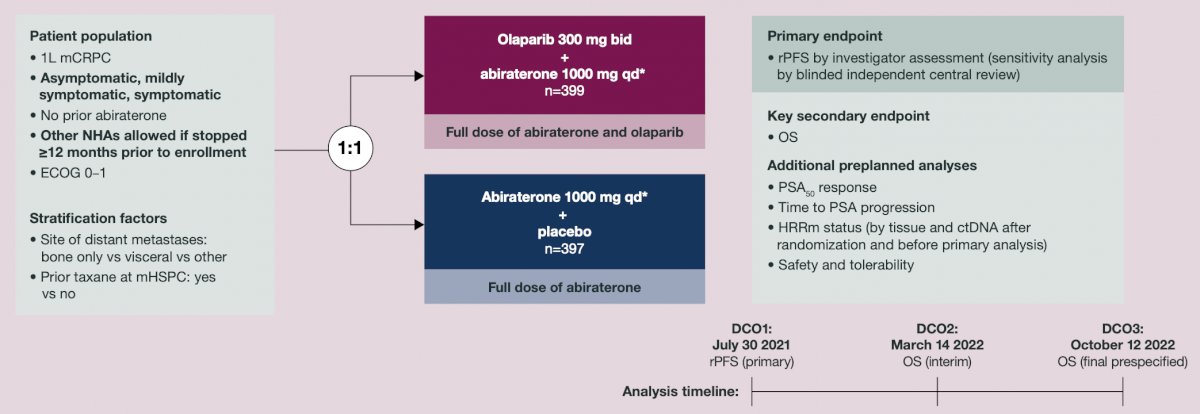
PSA response rate was the percentage of patients with a ≥50% decrease in PSA from baseline to lowest post-baseline result, confirmed by a second consecutive PSA result ≥3 weeks later. Time to PSA progression was time from randomization to first PSA progression per Prostate Cancer Working Group 3 criteria. Aggregated results from tumor tissue (FoundationOne®CDx) and circulating tumor DNA (FoundationOne®Liquid CDx) tests were used to classify HRRm and BRCA mutation status.
In this analysis, confirmed PSA response rate was 79.3% with abiraterone and olaparib vs 69.2% with abiraterone and placebo in the overall population, and ≥7% higher with abiraterone and olaparib vs abiraterone and placebo in each subgroup. The following shows olaparib + abiraterone increased PSA50 response rates compared with placebo + abiraterone in the ITT and in all biomarker subgroups, with the greatest effect observed in the BRCA mutated subgroups:

Time to PSA progression was prolonged with abiraterone and olaparib vs abiraterone and placebo in the overall and subgroup populations (BRCA mutated HR 0.14; 95% CI 0.07–0.25; HRRm HR 0.41; 95% CI 0.29–0.59):

Kaplan-Meier estimates show a benefit in time to PSA progression with olaparib + abiraterone in both the BRCA mutated and non-BRCA mutated populations, with the most pronounced difference between treatment arms in the BRCA mutated population:
Dr. Saad concluded his presentation by discussing the PSA analysis from PROpel with the following take-home messages:
- In this exploratory analysis, first-line mCRPC treatment with abiraterone and olaparib resulted in higher PSA response rates and prolonged time to PSA progression vs abiraterone and placebo in all patients, with more pronounced improvements in the HRRm and BRCA mutation subgroups
- While the PSA50 response rates were numerically higher and PSA progression was delayed with olaparib + abiraterone in the ITT and in all biomarker subgroups, the most pronounced effects were observed in the BRCA mutated subgroup
- These results are consistent with the primary rPFS benefit previously reported
Presented by: Fred Saad, MD, FRSC, Professor, Department of Surgery, Raymond Garneau Chair in Prostate Cancer, Université de Montréal, Montréal, QC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence 2022.EVIDoa2200043.



