(UroToday.com) The 2023 AUA annual meeting included an advanced prostate cancer session, featuring a presentation by Dr. Neal Shore discussing data from the ARAMIS rollover study assessing treatment duration and long-term safety and tolerability with darolutamide in nmCRPC. Darolutamide is a structurally distinct and highly potent androgen receptor inhibitor, with low blood-brain barrier penetration and limited potential for clinically relevant drug-drug interactions. In patients with nmCRPC, darolutamide significantly improved metastasis-free survival by ∼2 years and reduced the risk of death by 31% vs placebo, with a favorable safety profile (ARAMIS study).1 At the 2023 AUA annual meeting, Dr. Shore and colleagues reported the long-term safety and tolerability of darolutamide in the ARAMIS Rollover Study (NCT04464226).
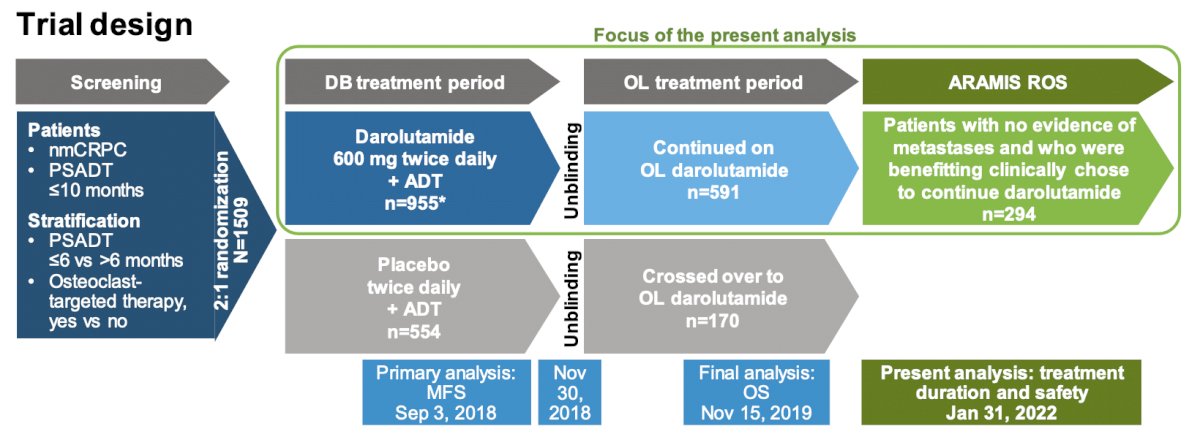
In ARAMIS, 954 patients received double-blind darolutamide 600 mg orally twice daily, of whom 591 patients continued on open-label darolutamide following the primary analysis. On completion of ARAMIS, 294 patients with no evidence of metastases and benefitting clinically from darolutamide entered the rollover study. Darolutamide treatment duration and safety are described for the 954 patients over the double-blind, double-blind + open-label, and double-blind + open-label + rollover study periods. The trial design for the ARAMIS rollover study is as follows:
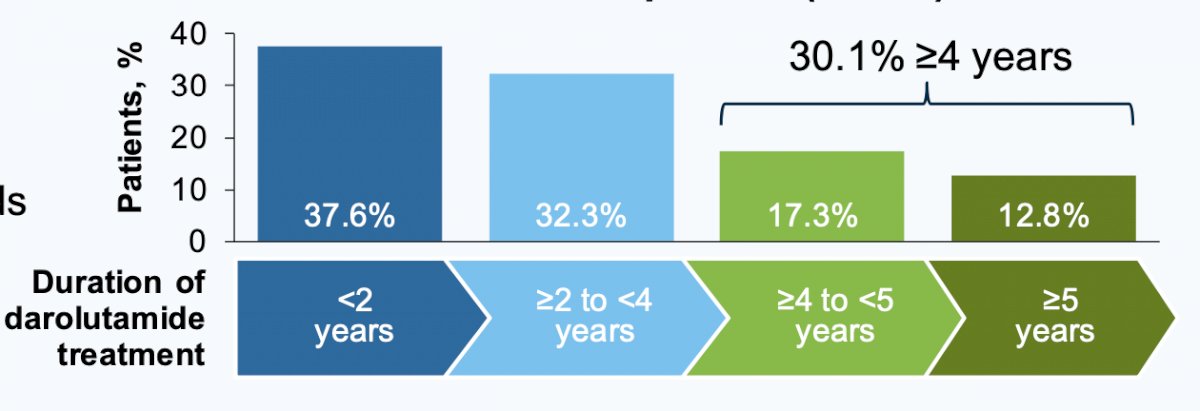
At the data cut-off of Jan 31, 2022, the median duration of darolutamide treatment was double-blind 1.5 years (range: 0.0–4.0), double-blind + open-label 2.1 years (range: 0.0–4.9), and double-blind + open-label + rollover study 2.8 years (range: 0.0–6.8). Of the 954 randomized patients, 32.3% received darolutamide treatment for ≥2 to <4 years, 17.3% for ≥4 to <5 years, and 12.8% for ≥ 5 years:
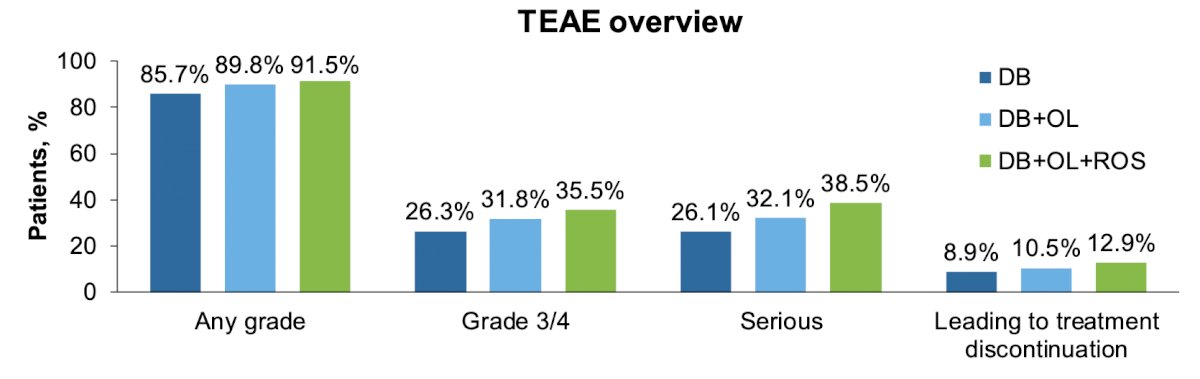
As expected, cumulative incidences of treatment-emergent adverse events increased slightly with longer observation time. For the double-blind, double-blind + open-label, and double-blind + open-label + rollover study periods, respectively, treatment-emergent adverse event incidences were:
- Any grade 85.7%, 89.8%, 91.5%
- Grade 3/4 26.3%, 31.8%, 35.5%
- Serious 26.1%, 32.1%, 38.5%
- Leading to treatment discontinuation 8.9%, 10.5%, 12.9%
Increases in incidences of treatment-emergent adverse events commonly associated with androgen receptor inhibition across the double-blind, double-blind + open-label, and double-blind + open-label + rollover study periods were mostly minimal. As follows is the cumulative incidence of treatment-emergent adverse events associated with androgen receptor inhibition over time in patients randomized to darolutamide (n = 954):
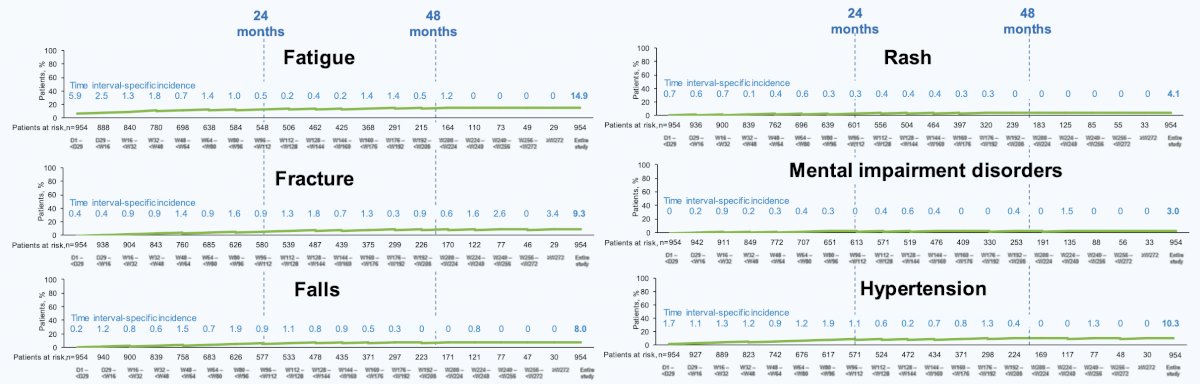
During the first 24 months of darolutamide treatment, incidences of most of these treatment-emergent adverse events were low and ≤2% different vs placebo, except for fatigue. The incidence and exposure-adjusted incidence rate of treatment-emergent adverse events associated with ARI therapy are as follows:

Dr. Shore concluded his presentation by discussing data from the ARAMIS rollover study assessing treatment duration and long-term safety and tolerability with darolutamide in nmCRPC with the following take-home messages:
- The ARAMIS Rollover Study extended the duration of darolutamide treatment and demonstrated the long-term safety of darolutamide in patients with nmCRPC
- Approximately 30% of patients with nmCRPC remained on darolutamide for ≥4 years, suggesting long-term clinical benefit
- Most adverse events occurred in the first 2 years of treatment, with small increments over time
- The favorable safety profile of darolutamide was maintained with long-term exposure
- No new safety concerns were observed in the rollover study
Presented by: Neal D. Shore, MD, FACS, Medical Director, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
Reference:- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246.


