(UroToday.com) The 2023 American Urological Association (AUA) annual meeting held in Chicago, IL between April 28 and May 1st, 2023, was host to an advanced prostate cancer moderated poster session. Dr. Benjamin Lowentritt presented the results of an analysis of PSA response and time-to-castration resistance among metastatic castrate sensitive prostate cancer (mCSPC) patients initiated on apalutamide, enzalutamide, or abiraterone acetate.
Second generation androgen receptor signaling inhibitors, such as apalutamide, enzalutamide, and abiraterone acetate, are approved in combination with androgen deprivation therapy as doublet therapy for patients with mCSPC. It has previously been demonstrated that an improved PSA response is associated with prolonged overall survival and a decreased rate of castration resistance development. The objective of this study was to describe a “real-world” experience of mCSPC patients treated with apalutamide, enzalutamide, and abiraterone acetate, with a focus on PSA response and development of castration resistance outcomes.
This study identified patients with mCSPC newly initiated on apalutamide, enzalutamide, or abiraterone acetate using data from 77 community-based urology practices between September 2018 and April 2022. In-office dispensing prescription data was used to identify the patient cohort, with index date defined as the first dispensation date. On-treatment PSA response was defined as:
- Post-index ≥90% decline in PSA (PSA90) from baseline (Index PSA obtained within 13 weeks of starting treatment- most recent PSA value used) AND
- Post-index decline to ≤0.2 ng/mL (PSA0.2) among those with baseline PSA >0.2 ng/ml.
Summary measures were used to describe the proportion of patients achieving a PSA response or progressing to castration-resistance, stratified by drug received. Survival analysis using Kaplan Meier curves were used to describe time-to-event outcomes.
This cohort included a total of 1,739 patients, with 589 apalutamide, 597 enzalutamide, and 553 abiraterone acetate users included. The mean index age was 76 years in all three groups. Mean baseline PSA levels were higher in the abiraterone acetate group (24 ng/ml), compared to the apalutamide and enzalutamide groups (19 ng/ml).
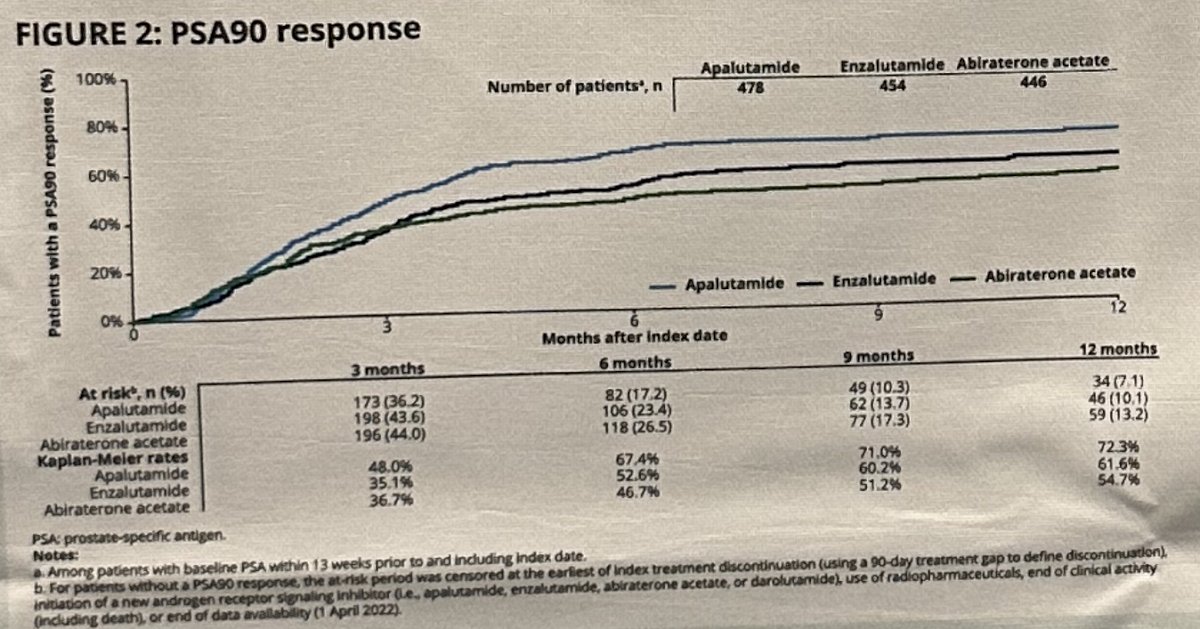
The outcome of PSA90 by 12 months was achieved in:
- Apalutamide group: 72%
- Enzalutamide: 62%
- Abiraterone acetate: 55%
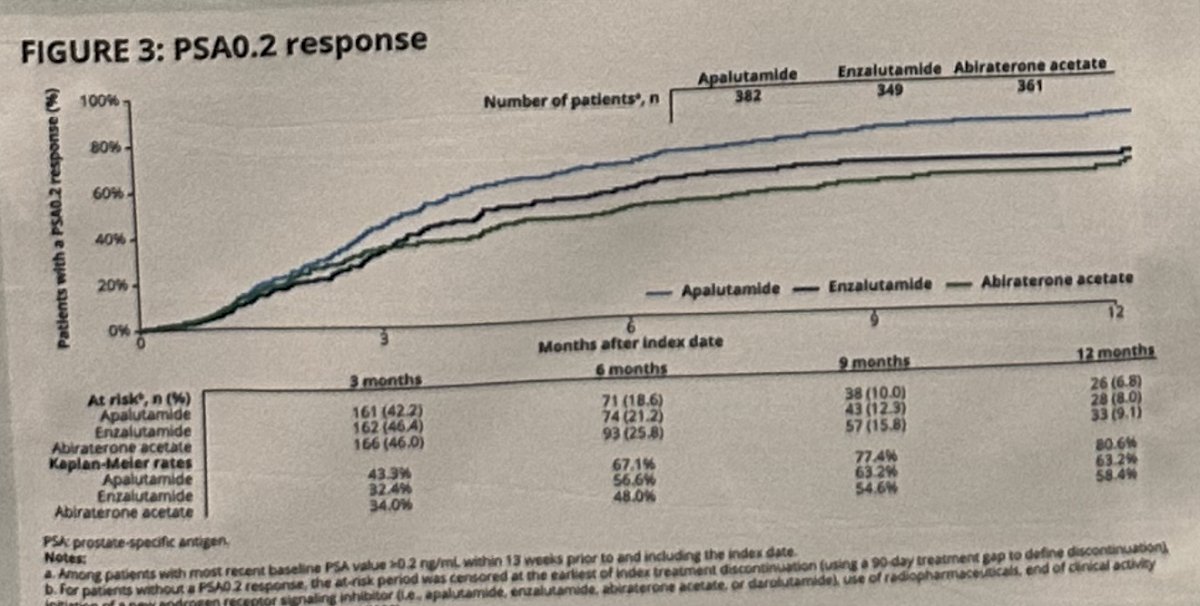
The outcome of PSA0.2 by 12 months was achieved in:
- Apalutamide group: 81%
- Enzalutamide: 63%
- Abiraterone acetate: 58%
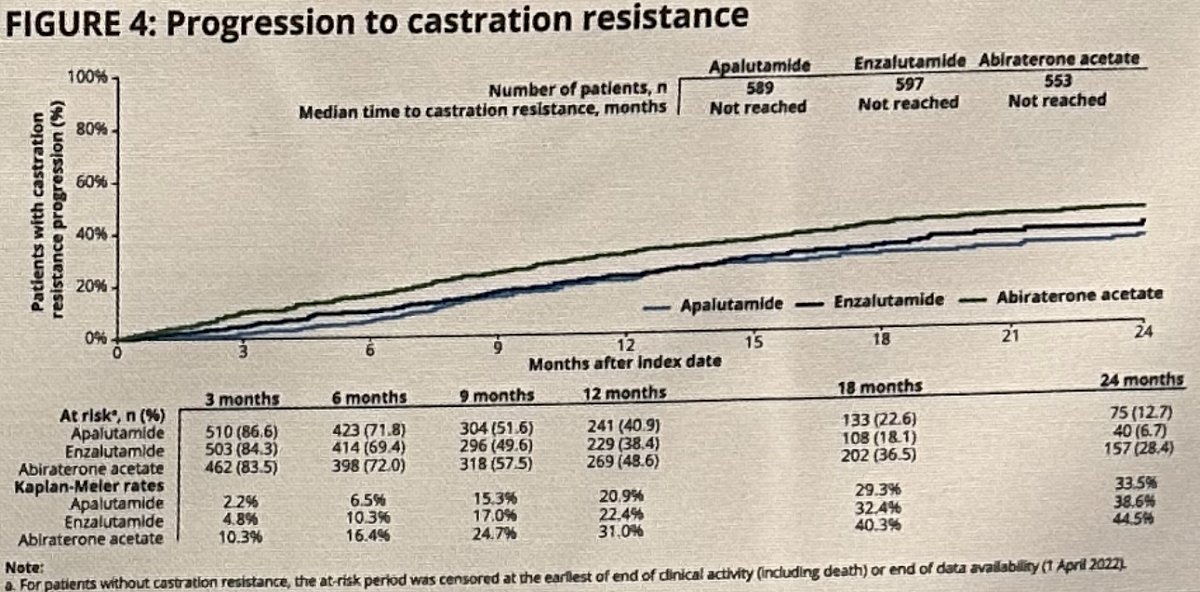
By 24 months, progression to castration resistance occurred in:
- Apalutamide group: 34%
- Enzalutamide: 39%
- Abiraterone acetate: 45%
Dr. Lowentritt concluded that this is among the first observational studies to describe real-world PSA responses and progression to castration resistance in mCSPC patients treated with ARSIs in US community urology practices. While differential PSA responses and time-to-castration resistance were observed among patients across the 3 drug groups, future studies that adjust for baseline patient differences/confounders are needed to ascertain these preliminary results.
Presented by: Benjamin H. Lowentritt, MD, FACS, Urologist, Chesapeake Urology Associates PA, Baltimore, MD
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023


