(UroToday.com) The 2023 American Urological Association (AUA) annual meeting held in Chicago, IL, was host to a plenary session with Dr. Neal Shore presenting the long-awaited results of EMBARK, a phase 3 randomized study of enzalutamide or placebo plus leuprolide acetate and enzalutamide monotherapy in high-risk biochemically recurrent prostate cancer.
Within 10 years of definitive therapy, 20-50% of prostate cancer patients experience disease recurrence characterized by rising PSA levels. There is a paucity of level 1 clinical data for the treatment of biochemically recurrent patients. Patients with high-risk biochemical recurrence are at increased risk of prostate cancer-specific mortality, with evidence from phase 3 clinical trials demonstrating that treatment intensification with androgen receptor signaling inhibitors (ARSI), such as enzalutamide, consistently improves patient outcomes across the prostate cancer continuum.
As such, the objective of EMBARK was to evaluate enzalutamide in combination with leuprolide acetate and enzalutamide monotherapy in patients with high-risk biochemical recurrence. The study design is summarized below. In brief, all patients had a PSA ≥1 ng/ml after RP or ≥2 ng/ml above nadir after primary EBRT, with a PSA doubling time (PSADT) of ≤9 months. Patients had no evidence of metastasis on conventional imaging and baseline testosterone was ≥150 ng/dL. Hormone therapy ≥9 months prior to enrolment was permitted. Patients underwent stratified randomization (by PSA level, PSADT, and prior hormonal therapy receipt) to one of three arms:
- Enzalutamide 160 mg (standard dose) + leuprolide acetate (blinded arm)
- Placebo + leuprolide acetate (blinded)
- Enzalutamide monotherapy (unblinded)
PSA was assessed at 36 weeks, and if patients had:
- PSA<0.2 Treatment was suspended at week 37 and PSA was monitored with treatment reinitiated if PSA rose again
- PSA>0.2 Treatment was continued
The primary endpoint was metastasis-free survival (MFS), assessed via blinded independent central review (BICR), in the enzalutamide + leuprolide versus leuprolide arms only. Key secondary endpoints included overall survival and safety outcomes.

Baseline characteristics are summarized below. Median age was about 69 years, with the majority of patients Caucasian (83%). Consistent with the stratified randomization technique, about 20% of patients in each arm had a PSADT ≤3 months, with the remaining 80% having a PSADT of 3 to 9 months. The median PSADT was approximately 5 months. 30% of patients had received prior hormonal therapy. Of note about half of the cohort had received both prior RP and RT.

At a median follow-up of 5 years, the combination of enzalutamide/leuprolide, versus leuprolide alone, demonstrated a significant improvement in MFS (HR: 0.42, 95% CI: 0.31 – 0.61, p<0.0001). The median MFS was not reached in either arm as of date.

Subgroup analysis demonstrated consistent benefits for this combination, irrespective of PSADT, baseline age, PSA, geographic region, receipt of prior hormonal therapy, or prior RP.

This combination arm also demonstrated an OS improvement (HR: 0.59, 95% CI: 0.38 – 0.90, p=0.0142). While the 95% CI upper bound does not cross 1 and the p-value is <0.05, the OS outcome is not yet mature and does not yet meet the pre-specified efficacy boundary of p<0.0001.

Similar benefits were seen for time to PSA progression (HR: 0.07, 95% CI: 0.03-0.14, p<0.0001), with the curve in the combination arm essentially remaining flat, indicating a very low number of events (n=8).

Time to first use of new antineoplastic therapy was similarly prolonged with this combination (HR: 0.36, 95% CI: 0.26 – 0.49, p<0.0001).
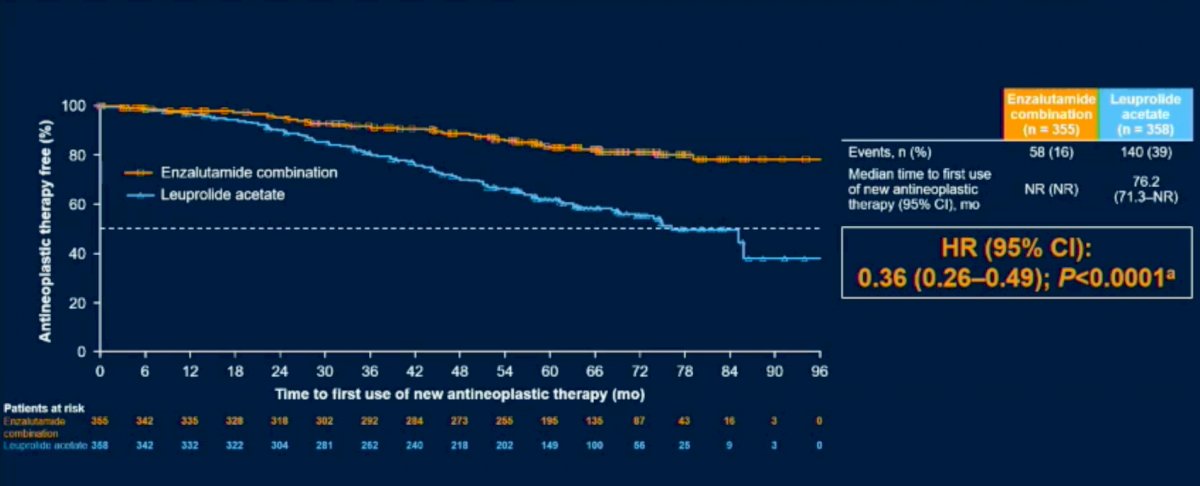
Next, comparisons between the enzalutamide monotherapy and leuprolide acetate monotherapy arms were performed. This demonstrated prolonged MFS in the enzalutamide only arm, with a HR of 0.63 (95% CI: 0.46 – 0.87, p=0.0049).

OS was also prolonged with enzalutamide monotherapy, albeit with the data remaining immature (140/271 anticipated events) and benefits were of modest clinical significance.

Similar to the enzalutamide/leuprolide versus leuprolide comparisons, time to PSA progression and time to first use of new antineoplastic therapy were prolonged in the enzalutamide versus leuprolide only comparisons.

Next, Dr. Shore compared the outcome of PSA<0.2 ng/ml at week 36 between the three arms. As seen below, this outcome was observed in 91% and 86% of patients in the enzalutamide/leuprolide and enzalutamide monotherapy arms, respectively, compared to 68% in the leuprolide only arm. The median duration of treatment suspension was, as expected, highest in the enzalutamide combination arm (20.2 months).

Given the mechanism of action of enzalutamide, an androgen receptor nuclear translocator inhibitor, a more significant testosterone suppression was observed in the arms including leuprolide acetate.

No new safety signals were observed with the combination of enzalutamide and leuprolide. Grade 3 or worse AEs were observed in 43-50% of patients across the three arms. The median treatment duration was longest with enzalutamide monotherapy (46 months), compared to enzalutamide combination (32 months) and leuprolide (35 months).

The most common AE leading to study drug discontinuation was fatigue (3% with enzalutamide combination versus 1% and 2% with leuprolide and enzalutamide monotherapy, respectively). Overall, the most common AEs (>15% of patients) for all treatment cohorts were hot flashes and fatigue.


Dr. Shore concluded his presentation of EMBARK with the following take home messages:
- In patients with high-risk biochemical recurrence, compared with leuprolide acetate, enzalutamide combination with leuprolide acetate demonstrated a statistically significant and clinically meaningful improvement in MFS (HR: 0.42, 95% CI: 0.30 – 0.61, p<0.0001).
- A consistent treatment effect was observed across pre-specified subgroups
- Significant delays in time to PSA progression and time to first new antineoplastic therapy were observed
- A trend towards improved survival in the interim analysis was noted; the study is ongoing for final analysis
- Enzalutamide monotherapy also demonstrated statistically significant and clinically meaningful improvements in MFS, time to PSA progression, and time to first antineoplastic therapy
- A trend toward improved survival in interim analysis
- No new safety signals were observed
As such, enzalutamide in combination with ADT, if approved in this setting, has the potential to become a new standard of care for patients with high-risk BCR with a PSADT <9 months and no evidence of metastasis on conventional imaging.
Presented by: Neal Shore, MD, FACS, Urologist, Director, CPI, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomized, double-blind, phase 3 trial. Lancet Oncol 2019 May;20(5):686-700.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019 Nov 10;37(32):2974-2986.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019 Jul 11;381(2):121-131.
EMBARK Trial Shows Promising Results for High-Risk Prostate Cancer Treatment, Enzalutamide's Impact on Metastasis-Free Survival - Neal Shore


