(UroToday.com) The 2023 AUA annual meeting included the International Prostate Forum, featuring a presentation by Dr. Isla Garraway discussing germline testing for prostate cancer in 2023. Prostate cancer is the most heritable cancer, with the following breakdown based on first degree of relative affected:

Genetic variants in germline DNA are associated with increased prostate cancer incidence and aggressiveness, and rare genetic variants affect only one gene (ie. BRCA2) but have a relatively strong correlation with prostate cancer. Single nucleotide polymorphisms (SNPs) occur across the genome, are quite common, and display a relatively weak association with prostate cancer. If an individual harbors many SNPs in their germline DNA, they have an increased lifetime risk of prostate cancer, which is represented by polygenic risk scores that include dozens to hundreds of SNPs.
Dr. Garraway highlighted that precision oncology treatments target specific genetic variants. Furthermore, there are three target-specific treatments based on genetic variants:
- Olaparib (PARP inhibitor): FDA approved for mCRPC with deleterious mutations of germline or somatic DNA repair genes (clinical trial: PROfound)1,2
- Rucaparib (PARP inhibitor): FDA approved for mCRPC with deleterious mutations of germline or somatic homologous recombination deficiency genes BRCA1/2 (clinical trial: TRITON2 and TRITON3)3,4
- Pembrolizumab (immune checkpoint inhibitor): FDA approved for deleterious mutations of germline or somatic mismatch repair genes or microsatellite instability high (MSI-H) (clinical trial: KEYNOTE 199)5
It is important to note that the only way to definitively identify individuals with heritable and potentially actionable genetic variants is through germline DNA testing. This is done by first obtaining normal epithelial or white blood cells obtained for DNA extraction and sequencing following selecting the desired next generation sequencing method. Dr. Garraway highlighted that the role of the urologist in germline testing is as follows:
- Identify the eligible patients
- Order the appropriate tests (germline and/or somatic)
- Return and interpretation of results
- Referral to medical oncology
- Referral for genetic counseling
- Clinical trial consideration
With regards to who should receive testing, Dr. Garraway notes that disease stage and family history are the key aspects. For disease stage, testing should be done on any man diagnosed with advanced stage disease, such as (i) spread to lymph nodes, (ii) spread to bones or other organs, and/or (iii) cancer that is confined to the prostate but is considered high or very high risk (based on tumor grade, physical exam, and/or PSA level at diagnosis). For family history, patients should be tested if they have:
- Family history of prostate and/or other cancers with early age of diagnosis
- Multiple family members diagnosed with cancer
- Known family history of genetic variants associated with increased risk of cancer (BRCA1/2)
Dr. Garraway provides the following flow diagram for ordering the genetic test at the VA where she practices:
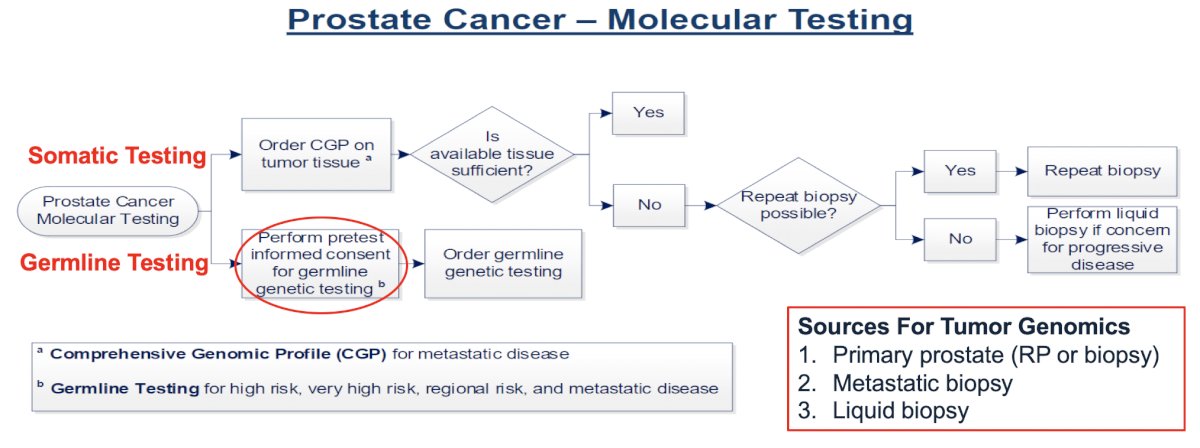
There are several options for obtaining informed consent for germline genetic testing, including genetic counseling, informational videos, and consent scripts embedded in the electronic medical record (particularly at the VA). Dr. Garraway highlights that there are several patient concerns about germline genetic testing, including privacy, discrimination, and loss of benefits. The Genetic Information Non-discrimination Act (GINA) was a US federal law signed in 2008, which safeguards individual and family privacy of genetic information. With this law, insurers are prohibited from determining eligibility, cost, coverage, or benefits of a health insurance policy based on an individual’s genetic information. However, GINA protections do not cover life, disability, or long-term care insurance, and GINA does not apply to individuals receiving their insurance through the federal government or military, which may result in veterans having reduction in Veterans Benefits (ie. service connection).
What can be helpful is having clinical care coordinators/navigators guide providers and patients through the consent, testing, and return of results process. In her clinic, she has a nurse practitioner with GU oncology experience that reviews charts to confirm patient eligibility, consent, and ensuring there is no duplicate testing. She engages with the urology provider, pathology points of contact, genetic counselor, and medical oncology, tracking results and alerting the care team of actionable pathogenic alterations.
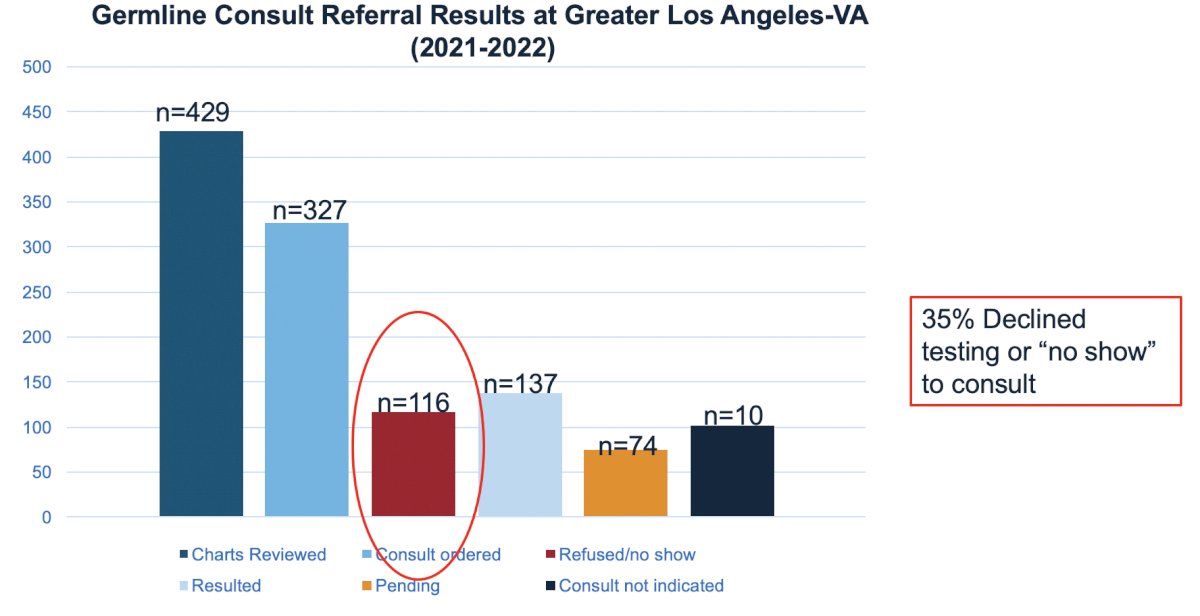
Work from the Great Los Angeles VA (2021-2022) highlights hesitancy for testing, noting that 35% of patients declined testing or “no-showed” their consult:
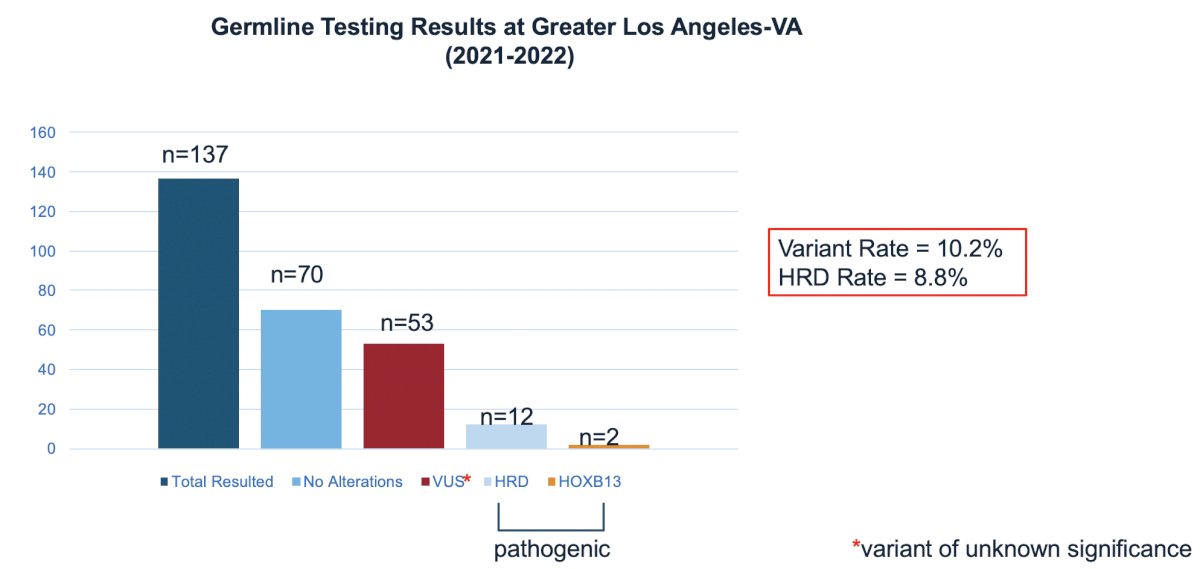
In this study, the variant rate was 10.2% and the HRD rate was 8.8%:
Dr. Garraway concluded her presentation by discussing germline testing for prostate cancer in 2023 with the following concluding messages:
- Germline testing is indicated in individuals diagnosed with high risk and/or metastatic prostate cancer, and those with a significant family history of cancer or genetic alterations associated with cancer
- The urologist has an important role in ensuring testing occurs by identifying candidates for testing and ensuring systems are in place to conduct testing, return of results, and appropriate referrals
Presented by: Isla P. Garraway, MD, PhD, UCLA Health, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Dec 10;383(24):2345-2357.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020 Nov 10;38(32):3763-3772.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023 Feb 16 [Epub ahead of print].
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol 2020 Feb 10;38(5):395-405.


