(UroToday.com) Dr. William Catalona started the debate regarding the renaming of Gleason 6 Prostate Cancer with the CON side of the debate, while Dr. Matthew Cooperberg followed with the PRO.
Dr. Catalona started things off by stating that the call to rename Gleason 6 (Grade Group 1) PCa is misguided. They call for wholesale reclassification of prostate “cancer” to “non-cancer.” One justification offered is precedent for renaming low grade bladder cancer and thyroid cancer (bladder PUNLMP, thyroid noninvasive follicular neoplasm with papillary-like nuclear features). But a major difference is that for bladder and thyroid, the diagnosis is made after the lesion has been completely excised and more aggressive histology has been ruled out. He also notes that another argument is that it doesn't matter whether low risk prostate cancer patients are treated because the ProtecT trial found no difference in mortality between patients managed with active monitoring versus radical treatment.
Similarly, he notes that the proponents of the change claim that renaming grade Group One will avoid the cancer label and believe that it will solve the overtreatment crisis. however, he argues that active surveillance is increasingly being adopted in the US and that overtreatment is being minimized. In 2021 about 60% of patients underwent active surveillance and it was at 80% within the VA. He notes that we can continue to improve the adoption of active surveillance in the US without having to rename the disease.
Another justification made by proponents of the change is that grade Group One will all but disappear with the increased use of MRI guided biopsies. This is to some degree 2 as the percentage of Grade Group 1 found on the initial screening trials were between 66 to 77%, while the more recent MRI targeted studies only found 9 to 40% GG1 disease. However, he feels this justification is irrational in the context of the individual patient. Even if the rate of grade Group One diagnosis were to decrease by 10% across the US it would amount to 30,000 grade Group One patients per year who would be erroneously told that they don't have prostate cancer. he feels that this would deceive patients - because 80% of primary prostate cancers harbor multiple tumor foci that can differ in grade.
He counteracts the claim that GG1 cannot metastasize as this data comes from series in patients who have undergone radical prostatectomy, which may have cured them of their disease thus precluding the development of metastases. The only way to determine the true biology of GG1 disease would require 20 plus years or more of untreated patients and no study to date answers this question. Even in the ProtecT trial, Still with only a 15 year median follow up, twice as many patients in the active monitoring group developed metastases.
Additionally looking at morphologic criteria, metastatic behavior is not the only criterion for malignancy. GG1 meets all of the morphologic criteria seen in higher grade cancers (ie invasive, loss of basal layer, perineural invasion, sometimes extracapsular extension). GG1 cancers can also have the same adverse genetic findings seen with higher grade tumors and with the same frequency, ie ERG mutations, recurrent point mutations, somantic DNA methylation of CpG islands.
From an MRI standpoint, it's a false assumption that visible lesions on MRI are always the most clinically relevant. He again pointed to a well known paper tracking the clonal origin of lethal disease in a single patient who died of prostate cancer and found that the clonal origin of the cancer that killed him was a 2 millimeter focus of Gleason grade Group 1 PCa – not his known high grade disease with lymph node metastases.
Looking at clinical behavior, GG1 tumors are reclassified on surveillance biopsies 30 to 50% of the time. the more extensively the biopsy specimens are involved with GG1 disease the more likely the tumor will express aggressive genomic biomarkers and behave aggressively. therefore the volume of GG1 may indicate an underlying more aggressive disease. In the ProtecT trial, 51% of patients who developed metastases and 46% who died of prostate cancer were originally diagnosed with GG1 disease! most prostate cancer deaths in patients with GG1 disease occur 12 to 25 years after treatment, so follow-up of 20+ years is important.
- In the ProtecT trial, “The intervals between the appearance of metastases and death continue to extend from 10 to 20 years"
- In Sweden, with long-term follow-up, 13% of GG1 patients managed with active surveillance died of prostate cancer
- In US SEER data, with 20-year follow-up, among patients who died of prostate cancer, more had GG1 disease than GG2+ disease.
Possible pitfalls of renaming GG1 as “non-cancer” –
- Active surveillance would only be used for patients with GG2 cancer for which surveillance protocols should be more intensive
- since compliance with biopsy for GG1 is already poor, it would be poorer with declassification
- Since poor compliance correlates with worse outcomes and underserved populations have poor compliance, declassification would increase disparities
- if some pathologists inaccurately grade GG2 as GG1, these patients would likely be compromised by not having appropriate surveillance
- GG1 would not be listed as cancer in tumor registries, and GG1->GG2 would be listed as new cancer
- Volume of disease would be compromised in risk-stratifying patients
His way forward:
- Acknowledge the overtreatment issue and deal with it by developing evidence based guidelines to increase the appropriate adoption of active surveillance and the quality of surveillance
- continue to integrate traditional risk assessment parameters
- increase patient and physician education about the appropriate management of low risk prostate cancer
- apply implementation science (methods and procedures to induce urologists to “do the right thing”)
- But, do NOT rename GG1 prostate cancer
On that note, Dr. Cooperberg took over and titled his talk “Its Time to Rename Gleason 6.” Cancer (from the Latin) implies insidious growth and spread – and nomenclature matters.
In a recent paper and call to action by him and colleagues (Eggener et al. JCO 2022), they make the following key points:
- Gleason 6 is extremely prevalent. Diagnosis is often incidental to BPH and other factors related to BPH (ie elevated PSA, urology visits)
- Gleason 6 never metastasizes
- Gleason 6 has few, if any, molecular hallmarks of cancer
- AS is still done highly inconsistently
- “Gleason 6” as a non-cancer would still require surveillance
- The harm: benefit ratio of screening is improved the less we overdiagnose low-grade disease
Ross et al. (Am J Surg Path 2012) noted that prostate cancers with Gleason 6 or less do not appear to metastasize to lymph nodes. Upgrading to higher grade disease is required for metastatic disease.
In agreement with Dr. Catalona, Dr. Cooperberg acknowledged that GG1 does have some genomic changes associated with malignancy – but so does histologically normal tissue! (Erickson et al. Nature 2022).
He then looked at the hallmarks of disease and asked if Gleeson 6 meets those hallmarks.
- self-sufficiency in growth signals – NO
- insensitivity to growth inhibition signaling – NO
- resistance to apoptosis signals – NO
- unlimited replicative potential – NO
- sustained angiogenesis – NO
- tissue invasion and metastasis – NO
He notes that the biology of PCa development is a continuum – and the current placement of the diagnosis of “cancer” is an arbitrary line. It could be easily shifted to the right.
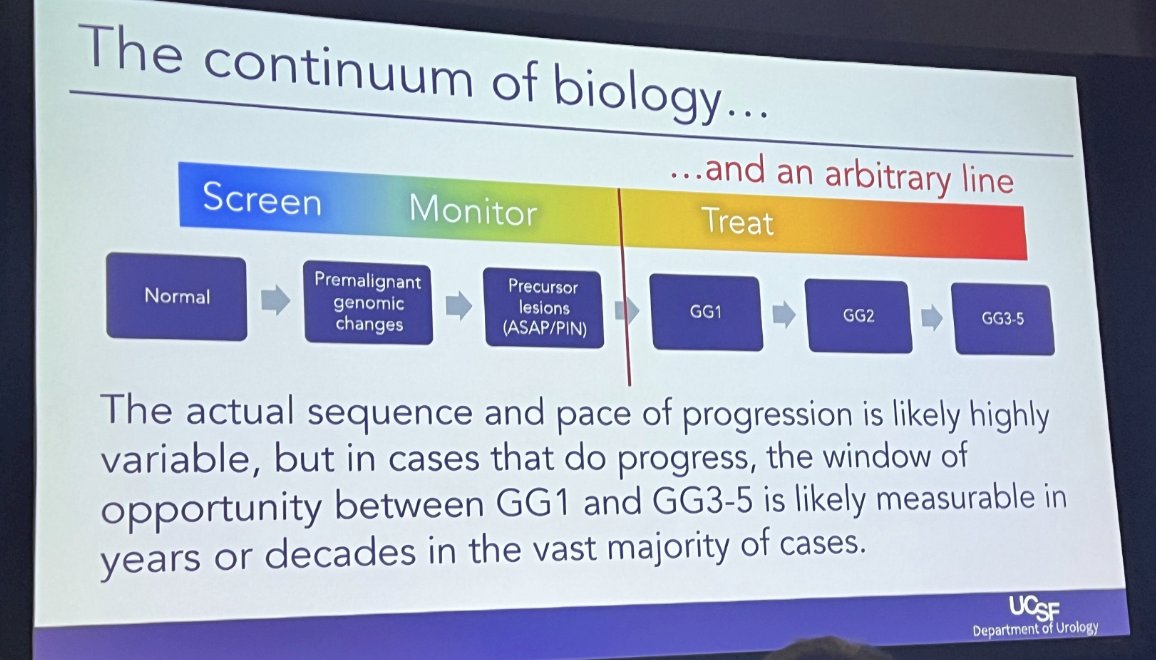
He notes that there is likely a window of opportunity to identify GG2-5 disease years to decades after when GG1 would have been diagnosed without losing opportunity for cure.
As it were, we are already using multiple biomarkers to help avoid diagnosing GG1 at the time of biopsy – including tissue, genomic, imaging biomarkers. the use of these biomarkers is working and we are now finding much lower rates of GG1 disease (down to about 20% in the AQUA registry). We are also making progress on active surveillance adoption which Dr. Catalona also mentioned – currently around 60% adoption. the impact of these changing practices in the US are that prostate cancer mortality has nadired at around 20 deaths/100,000 males.
Dr. Cooperberg notes that the reversal in the USPSTF recommendations was due to the fact that AS helped mitigate overtreatment. therefore he feels that the greatest impact at the population level that we can have may be to convince more primary care physicians to embrace early baseline PSA testing - but this may only be possible if we solve over diagnosis and over treatment.
He summarizes the counter arguments as well as his arguments on the following slide:

He notes that this push for renaming is not new. Dr. Ian Thompson published on the topic back in 2009, and a group of urologists/pathologists/Radiation oncologists met in 2023 to push for this again.
In a survey of PCa specialists worldwide on the topic, Saoud et al. (EU Focus, in press) found that 39% thought it was a good idea while 30% were uncertain.
- those most enthusiastic for a name change were clinicians, younger (<40), and fellowship trained
- those least enthusiastic were older clinicians (42% > age 60) and pathologists
His concluding remarks are the following:
- GG1 is an exceptionally common feature of aging, and in 2023 nearly universally represents overdiagnosis, effectively an incidental adverse effect of screening intending to find GG >= 2 cancers
- we are tacitly accepting non diagnosis of GG1 via MRI and liquid markers anyway already
- GG1 is never a lethal diagnosis. it might precede diagnosis of “real” cancer, but so can a negative biopsy. No one is saying GG1 is “normal.”
- Renamed GG1 would still require surveillance, but would rarely (not to say never) merit radical treatment
- this change would dramatically improve the benefit to harm ratio for screening, and likely improve primary care uptake of screening, with mortality benefits far more than outweighing missed progression events.
- Matthew R. Cooperberg, MD, MPH, Professor of Urology; Epidemiology & Biostatistics, Helen Diller Family Chair in Urology, University of California, San Francisco, San Francisco, CA
- William Catalona, MD, Professor of Urology, Northwestern University, Evanston, IL
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023


