(UroToday.com) During the 2023 American Urological Association’s included a late breaking abstract session, where Dr. Scott Eggner presented updated results examining progression-free survival (PFS) from PRESTO, a Phase 3 Randomized Study of Androgen Annihilation for High-Risk Biochemically Relapsed Prostate Cancer.
When patients experience biochemical recurrence (BCR) after radical prostatectomy, PSA doubling time (PSADT) is utilized to assess risk of development of distant metastases; men with short PSADT are deemed high risk for metastases and prostate cancer specific mortality. Intermittent androgen deprivation therapy (ADT) is included in treatment of BCR. Dr. Eggner reviewed that prior studies have demonstrated that abiraterone acetate and prednisone, along with apalutamide, have provided a longer duration of progression free survival (PFS) without quality of life detriments in men with BCR. Therefore, he and his study authors hypothesized that intensification of androgen deprivation may provide a more durable PSA suppression, allowing for longer time intervals off of treatment.
The trial structure is noted below:

The primary objective of the trial was PSA-PFS, defined as nadir + 2 ng/mL during treatment or >0.2 ng/mL following treatment, confirmed on repeat measurement (>2 weeks). Secondary objectives included PSA-PFS in the testosterone evaluable population (T > 50 ng/mL), time to recovery of serum testosterone (T > 50 ng/mL), safety profile, 36-month PSA-PFS survival rate, metastasis-free survival (MFS), time to castration resistance, short and long-term patient reported quality of life.
Of note, Dr. Eggner stressed that each intervention arm was compared against the control – the trial was not powered to compare each arm against each other.
He reviewed the characteristics of the trial population, noted below:


Of 643 patients screened, 503 were randomized:

PSA-PFS in the ADT + apalutamide arm was significantly prolonged.

The combination arm, including ADT + apalutamide + abiraterone, also significantly prolonged PSA-PFS.

Next, Dr. Eggner presented PSA-PFS stratified by PSADT:

The number of subject with testosterone recovery were similar in each arm:

There was no difference in time to testosterone recovery:

From a safety standpoint, arm B did have more Grade 3 and 4, as well as serious adverse events. Most commonly, patients experienced hypertension.
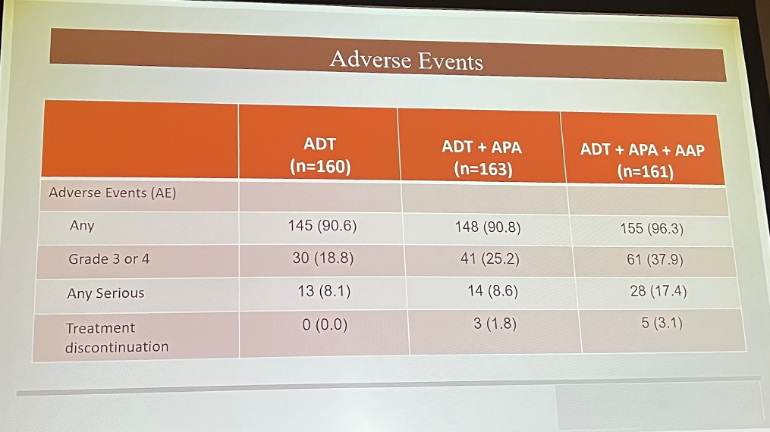

Dr. Eggner did note several limitations of the trial data presented, including that the primary endpoint was PSA-driven, as opposed to driven by evidence of metastases. Additionally, the trial began enrolling prior to utilization of PSMA-PET scanning.
He concluded his presentation by noting the following:
- The addition of apalutamide to ADT for 1 year leads to significantly prolonged PSA-PFS, without adverse impact of time to testosterone recovery
- The safety profile is consistent with prior studies
- There is no major benefit to adding abiraterone and prednisone to apalutamide
- Toxicity was increased
- Study was not designed or powered to compare arms with each other
- Ongoing follow up to evaluate patient-reported outcomes, time to castration resistance, and metastasis-free survival will provide further information
Presented by: Scott Eggner, MD, University of Chicago, Chicago, IL
Written by: Ruchika Talwar, MD, Urologic Oncology Fellow, Vanderbilt University Medical Center, during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:- Pound CR, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999
- Crook JM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy [published correction appears in N Engl J Med. 2012


