(UroToday.com) The 2023 American Urological Association (AUA) annual meeting held in Chicago, IL was host to the International Prostate Forum, with Dr. Thomas Boike discussing the urologist’s role in the field of theranostics.
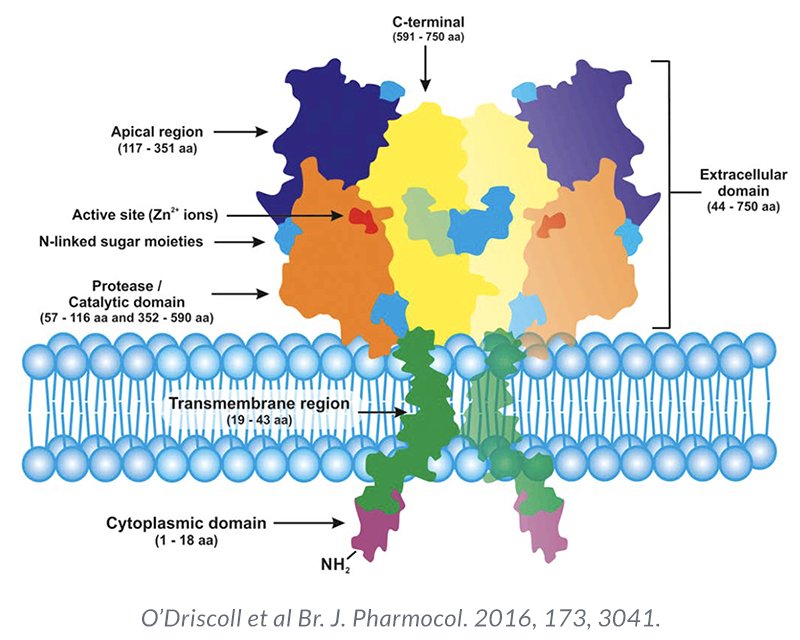
Theranostics is a form of diagnostic testing employed for selecting targeted therapy, and, as such, has both diagnostic and therapeutic components. Prostate-specific membrane antigen (PSMA) is a transmembrane protein expressed by the epithelial cells lining the proximal renal tubules, salivary glands, small bowel, as well as the prostate. Expression of this transmembrane protein is upregulated in prostate cancer cells by up to a 1000-fold. The PSMA gene is located on the short arm of chromosome 11 in a region that is not commonly deleted in prostate cancer, thus making it highly prevalent in all forms of prostate cancer, including some castrate resistant forms. Importantly, the relatively poor expression of PSMA in other organs allows for enhanced targeted imaging in prostate cancer patients.
TheraP was the first randomized study to evaluate 177Lu-PSMA-617 versus cabazitaxel for men with mCRPC in the post-docetaxel setting. In this open label, phase II trial, 200 men were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax≥20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. Overall, 200 patients were randomized 1:1 to 177Lu-PSMA-617 at a dose of 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every 3 weeks for up to 10 cycles. Patients were stratified based on disease burden and prior anti-androgen therapy. Of note, about 1/3 of patients who had registered for the study (91/291) were ineligible prior to randomization, either because of low PSMA expression or FDG discordant disease. The primary endpoint of this study was a PSA decline of 50% (PSA50) and secondary endpoints included PSA-PFS and OS. After a median follow up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR 0.63, 95% CI 0.46 to 0.86) and had a much higher PSA50 rate (66% vs 37%):
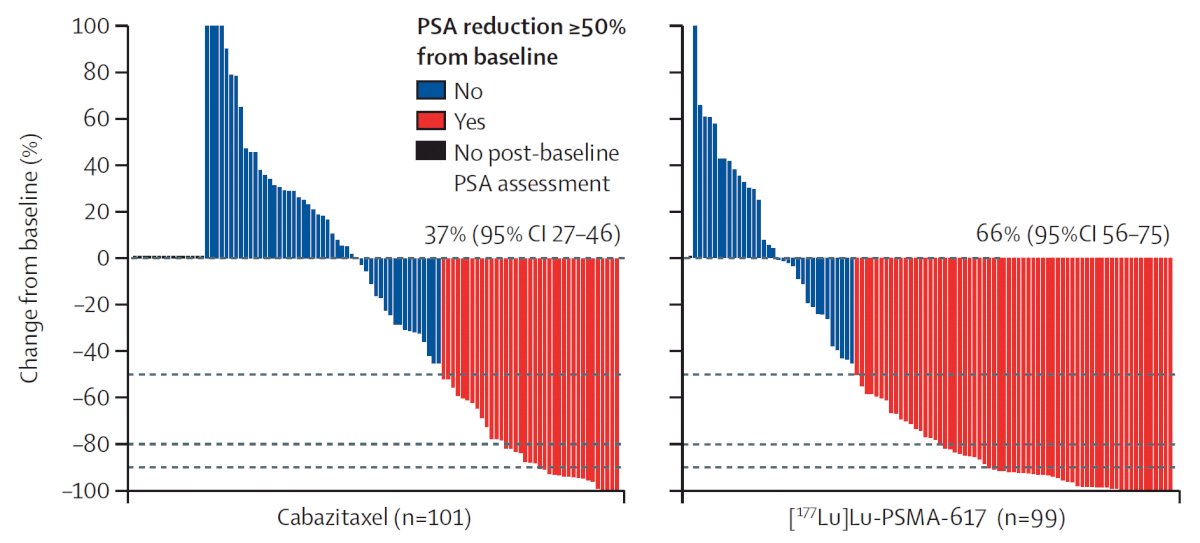
Additionally, there were superior RECIST response rates (33% versus 53%). In terms of the AE/safety profile, Grade 3/4 toxicity was seen in 54% of men on cabazitaxel compared to 35% of patients who received 177Lu-PSMA-617. Rates of thrombocytopenia, dry mouth, and dry eyes were seen more frequently in patients receiving 177Lu-PSMA-617, as expected due to normal PSMA expression in the salivary and lacrimal glands.
Updated analysis was recently presented at ASCO 2022 after a median follow-up of 36 months and PFS continued to favor the 177Lu-PSMA-617 arm (HR 0.62, 95% CI 0.45 to 0.85). There were no significant differences in restricted mean survival time OS between the two arms (19.1 months in 177Lu-PSMA-617 arm versus 19.6 months in cabazitaxel arm, 95% CI for difference: -3.7 - +2.7). Notably, OS was significantly worse in the 61/90 excluded patients with evaluable data, with restricted mean survival time OS of 11.0 months in these patients despite 48% and 5% eventually receiving cabazitaxel and 177Lu-PSMA-617, respectively.1
Following on the heels of TheraP, VISION was an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with a next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy. Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans, with PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. Standard of care treatments were at the discretion of the treating investigator; however, cytotoxic chemotherapy, immunotherapy, and radium-223 were explicitly excluded. Most patients received alternative androgen-directed therapies while others received palliative radiotherapy and glucocorticoids.
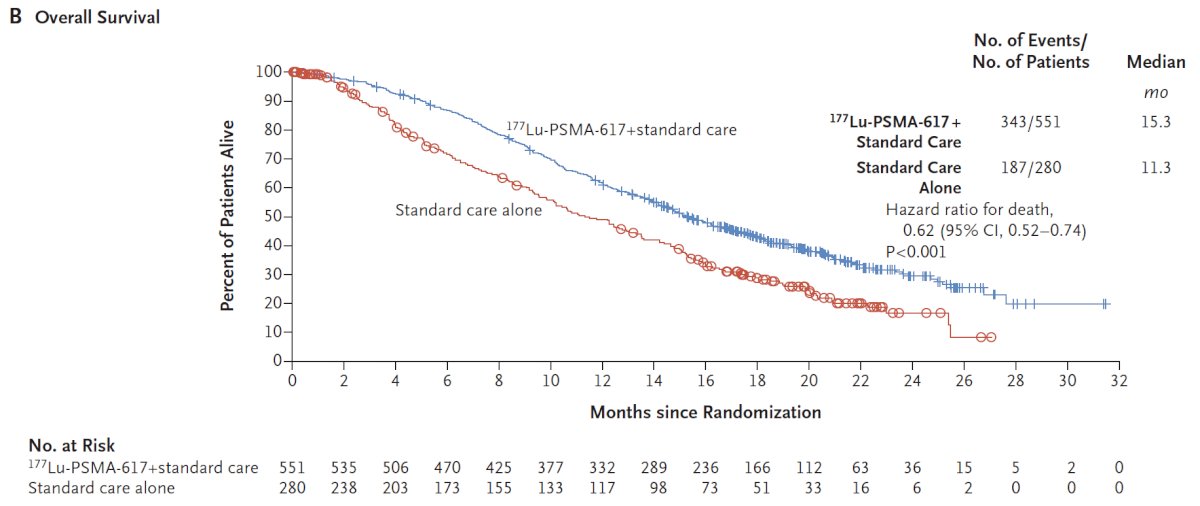
The authors assessed two alternate primary endpoints: (i) rPFS using the Prostate Cancer Working Group 3 (PCWG3) criteria by independent central review and (ii) OS. VISION enrolled 831 patients (2:1 ratio) between June 2018 and October 2019. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved OS by a median of 4.0 months (median OS: 15.3 vs 11.3 months; HR 0.62, 95% CI 0.52 to 0.74; p < 0.001, one-sided), compared to standard of care alone, in the overall cohort of all randomized patients (n=831):
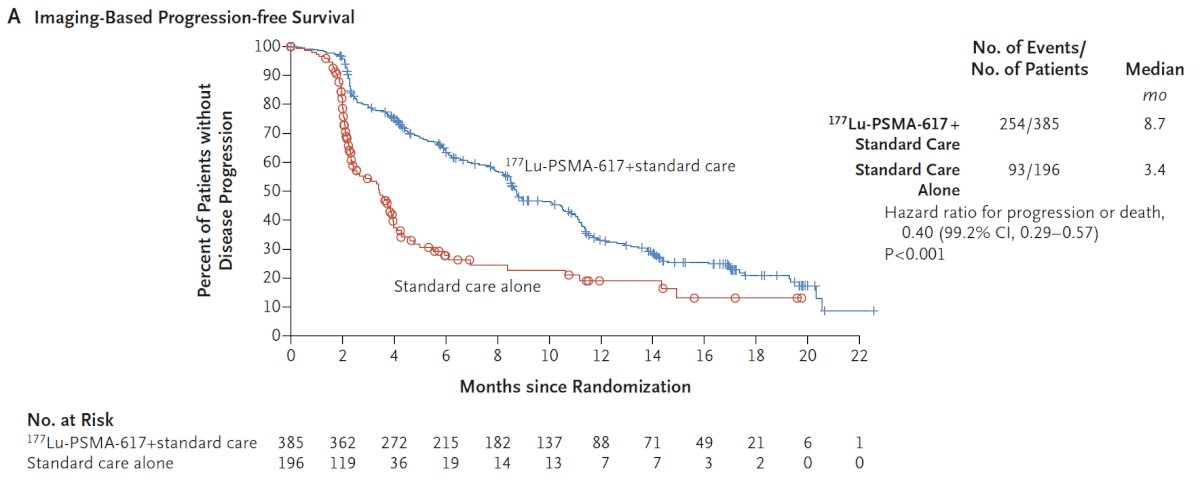
With regards to the other primary endpoint of rPFS, treatment with 177Lu-PSMA-617 + standard of care significantly improved rPFS by a median 5.3 months (median rPFS, 8.7 vs 3.4 months; HR 0.40, 99.2% CI 0.29 to 0.57; p < 0.001, one-sided):
The addition of 177Lu-PSMA-617 to standard of care statistically significantly improved all key secondary endpoints, including ORR (29.8% vs 1.7%), disease control rate (89.0% vs 66.7%) and time to first symptomatic skeletal event (median time: 11.5 vs 6.8 months; HR 0.50, 95% CI 0.40 to 0.62). Patient reported outcomes as evaluated by the FACT-P and Brief Pain Inventory (BPI) scores favored the 177Lu-PSMA-617 arm with delays in time to worsening of 7.3 and 11.4 months, respectively. While a higher rate of high-grade (grade 3-5) treatment-emergent AEs was observed with 177Lu-PSMA-617 (28.4% vs 3.9%) at the time of initial reporting, overall therapy was well tolerated. It bears note that treatment exposure was more than three times longer in the 177Lu-PSMA-617 group than in the control group. Adjusted safety analysis, accounting for a longer safety observation due to longer rPFS in patients receiving 177Lu-PSMA-617, revealed a comparable incidence of treatment-emergent AEs between the arms.2
Dr. Boike noted that these two trials relied on 68Ga-PSMA-11 for patient selection. Accordingly, he highlighted an abstract from his group presented at this conference (AUA23) that evaluated early outcomes from Lu-177 Vipivotide Tetraxetan treatment in patients selected for therapy using 18F-DCFPyL or 68Ga-PSMA-11 PET scans. PSA treatment responses were noted to be similar to those reported in the VISION trial irrespective of whether patients were selected using 18F-DCFPyL or 68Ga-PSMA-11 PET-based imaging, supporting current guidelines recommendations for the use of either in clinical practice for treatment selection purposes.

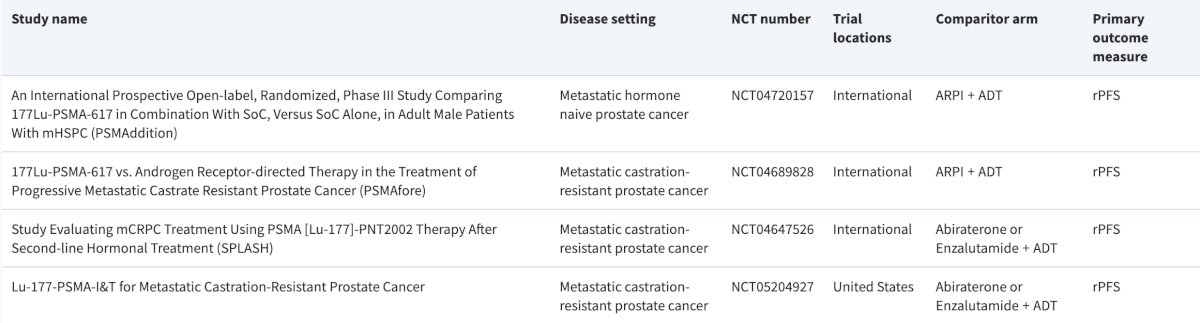
Dr. Boike concluded by highlighting select trials in this disease/treatment space:
- PSMAddition: An International Prospective Open-label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination with SoC, Versus SoC Alone, in Adult Male Patients With mHSPC
- PSMAfore: 177Lu-PSMA-617 vs. Androgen Receptor-directed Therapy in the Treatment of Progressive Metastatic Castrate Resistant Prostate Cancer
- SPLASH: Study Evaluating mCRPC Treatment Using PSMA [Lu-177]-PNT2002 Therapy After Second-line Hormonal Treatment
- Lu-177 PSMA-I&T for Metastatic Castration-Resistant Prostate Cancer
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


