(UroToday.com) The 2023 American Urological Association (AUA) annual meeting held in Chicago, IL between April 28 and May 1st, 2023, was host to the International Prostate Forum, with Dr. Peter Carroll discussing somatic testing
Somatic tumor testing refers to testing the DNA or other generic material of tumor tissue (prostate, lymph node) as opposed to a patient’s healthy germline tissue.
- Genetic testing generally refers to testing the DNA of a cell to look for genetic mutations
- Genomic testing refers to looking at other genetic material (RNA, for example) inside the cell
It is currently estimated that ~55% of “experts” use prostate cancer genomic classifiers in clinical practice:1

Based on the current evidence, Dr. Peter Carroll utilizes somatic testing for patients with:
- Metastatic cancer
- Intermediate risk, localized disease
- When considering concurrent ADT use for patients set to receive radiation therapy
- Patients with metastatic disease, especially at relapse
The current prostate cancer NCCN guideline suggests that somatic testing may complement currently available risk stratification tools, such as the NCCN risk classification system, CAPRA, STAR-CAP, and the MSKCC risk stratification tools.
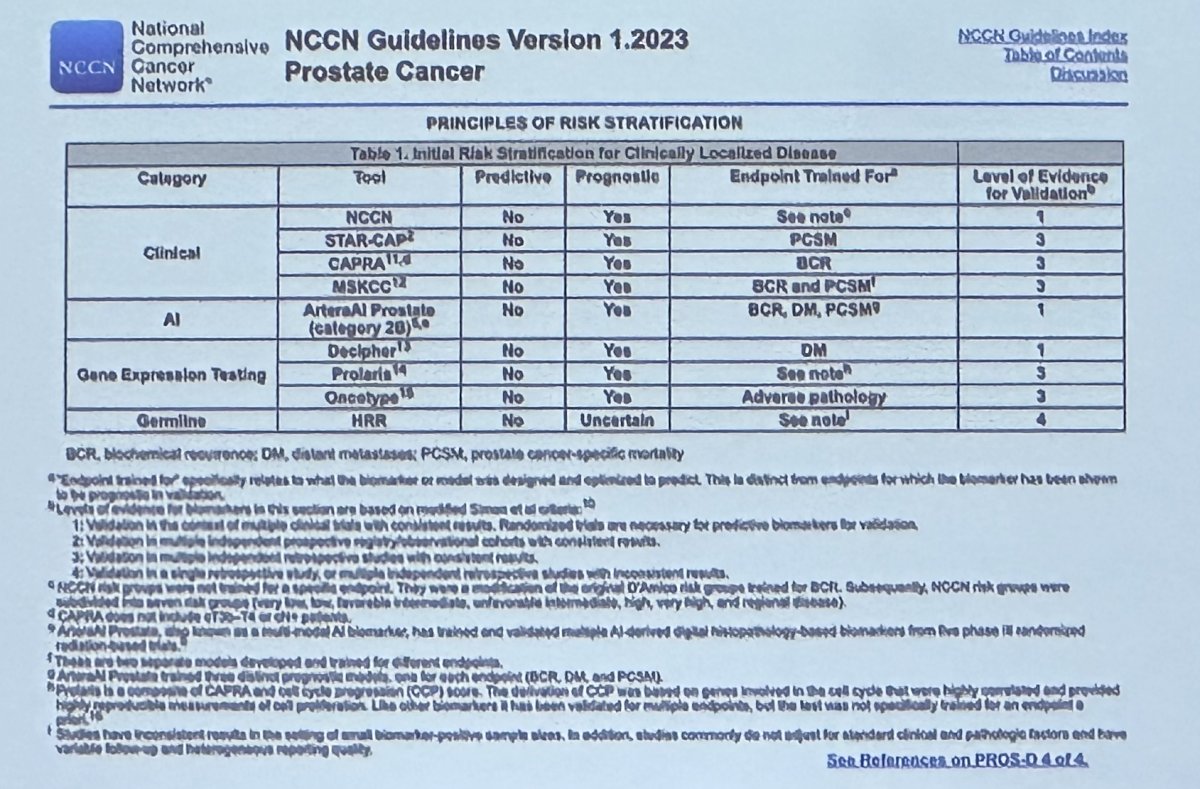
Currently, available somatic tests in this setting, among many others, include:
- Decipher
- Ki-67
- Oncotype
- PTEN
- Prolaris
For patients being considered for active surveillance (AS), there is strong clinical rationale for adding genomic prostate score (GPS) to the CAPRA risk stratification system. As demonstrated below, an increased GPS, a surrogate of increased tumor mutational burden, was shown to independently predict the risk of unfavorable pathology in patients undergoing a radical prostatectomy (RP), irrespective of CAPRA score.2
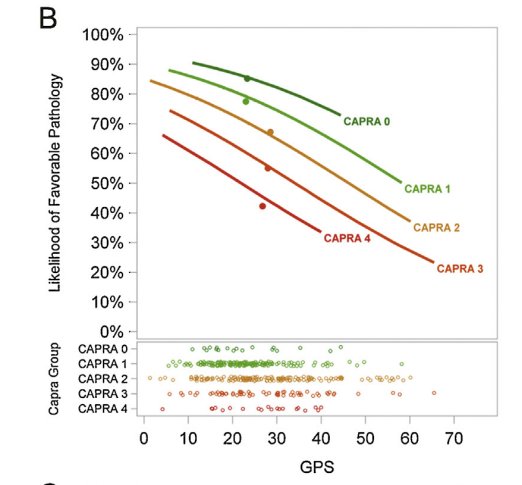
Similarly, Oncotype GPS was shown to be significantly associated with the odds of adverse pathology and PSA recurrence after delayed RP among AS patients.

However, at the current time, there does not appear to be an Oncotype GPS score cut-off that entirely excludes or predicts the occurrence of these adverse endpoints:

While somatic testing may have numerous benefits for clinical decision-making, Dr. Carroll emphasized that we must not ignore other commonly available biomarkers:
- Age
- PSA density
- PTEN staining
- mpMRI findings
- Volume of GG1 disease
- Pattern 4 histologic subtype Correlates with genomic classifier results
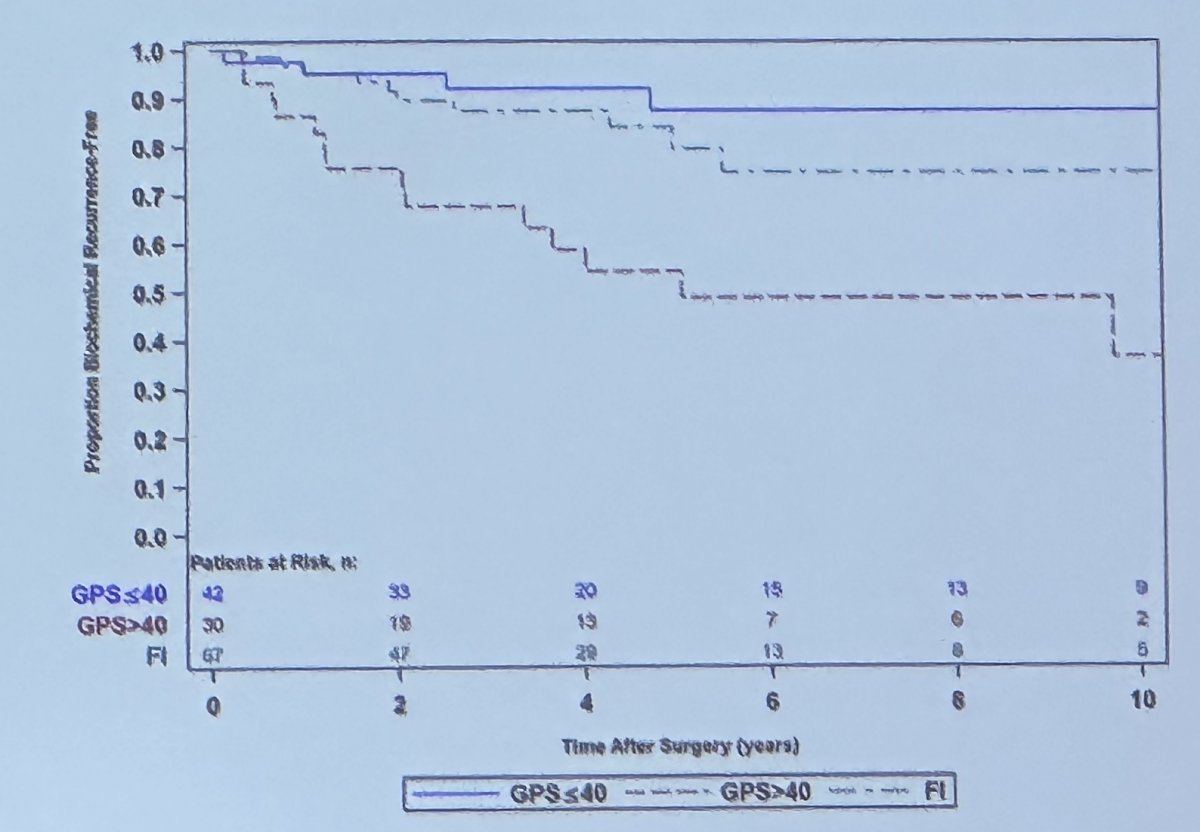
What about the use of GPS in higher risk patients? GPS is generally used for low and favorable intermediate risk patients. A GPS score of >40 among patients with intermediate risk disease (~40% of such patients) is associated with BCR rates approaching those of high-risk patients as demonstrated below:
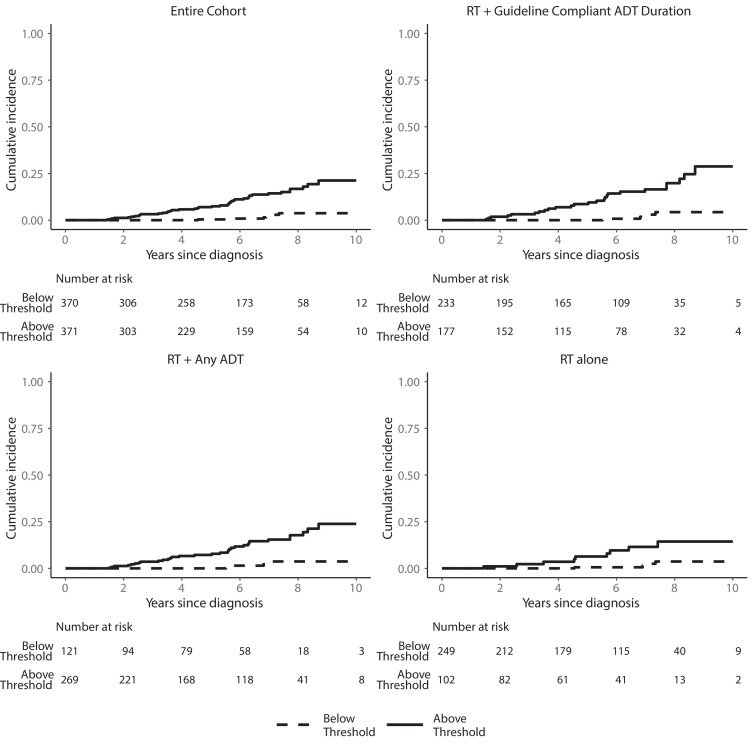
Can we use these genomic biomarkers for predictive, as opposed to prognostic only purposes? One such tool may be the clinical cell-cycle risk score for patients undergoing RT +/-ADT. The CCR score combines the UCSF CAPRA score and the cell cycle progression (CCP) molecular score and has been validated to be prognostic of disease progression for men with prostate cancer. A retrospective, multi-institutional cohort study of men with localized NCCN intermediate-, high-, and very high-risk prostate cancer (n=741) demonstrated that among patients with below-threshold CCR scores, there was minimal clinical benefit to adding concurrent ADT to RT (2% absolute MFS benefit). As such, this CCR score could be used as a predictive biomarker to select patients for RT intensification with ADT.3
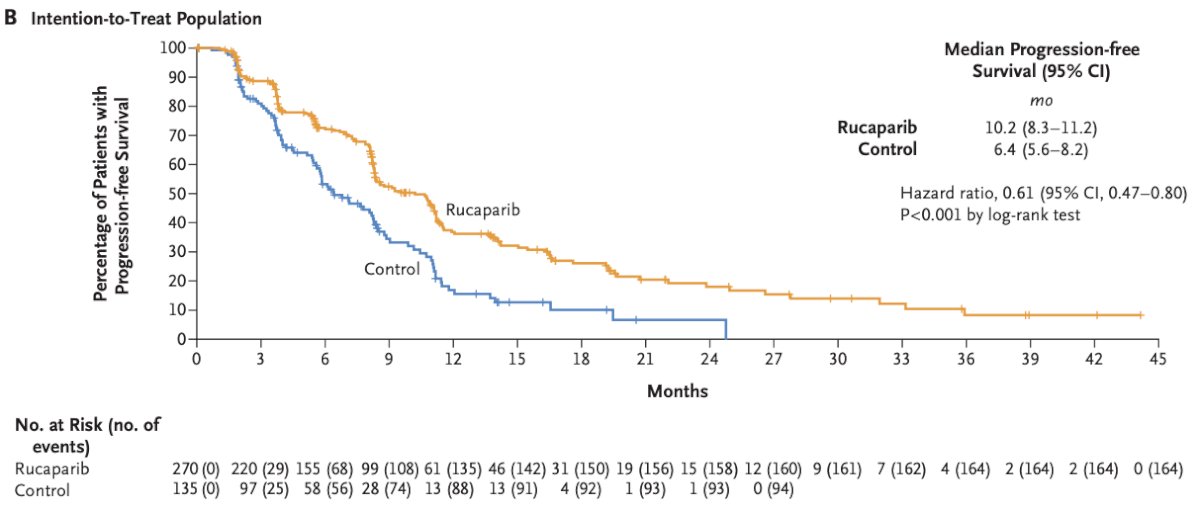
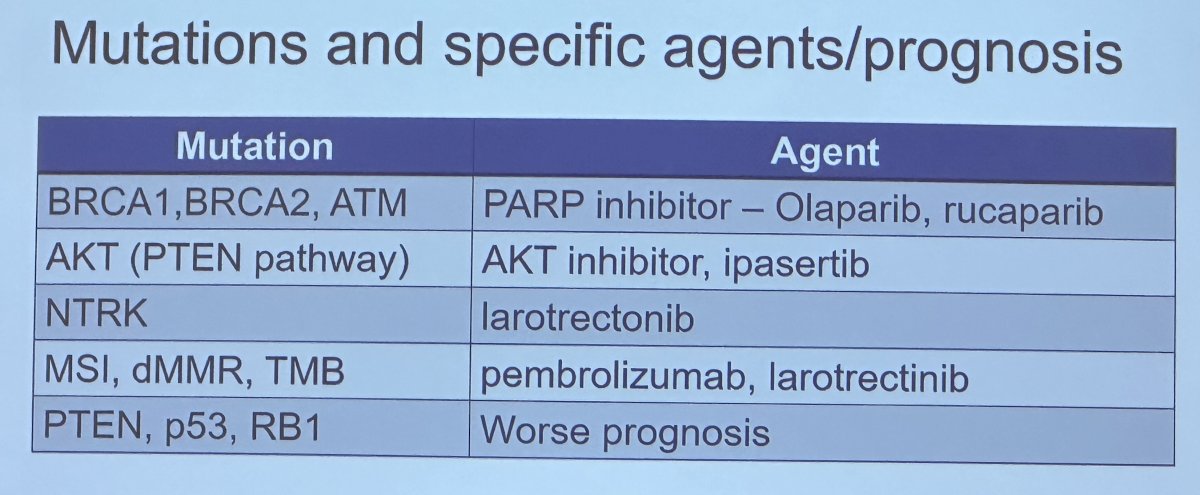
The predictive role of genomic testing for treatment selection in metastatic patients is more well-established. Homologous recombination repair (HRR) mutations (i.e., BRCA1/2) play a significant role in PARP inhibitor treatment selection, with Dr. Carroll highlighting the recent TRITON-3 trial that demonstrated rPFS benefits for rucaparib (median: 11.2 versus 6.4 months) in patients with mCRPC and BRCA1/2 or ATM alterations who had disease progression on a prior androgen receptor signaling inhibitor.4
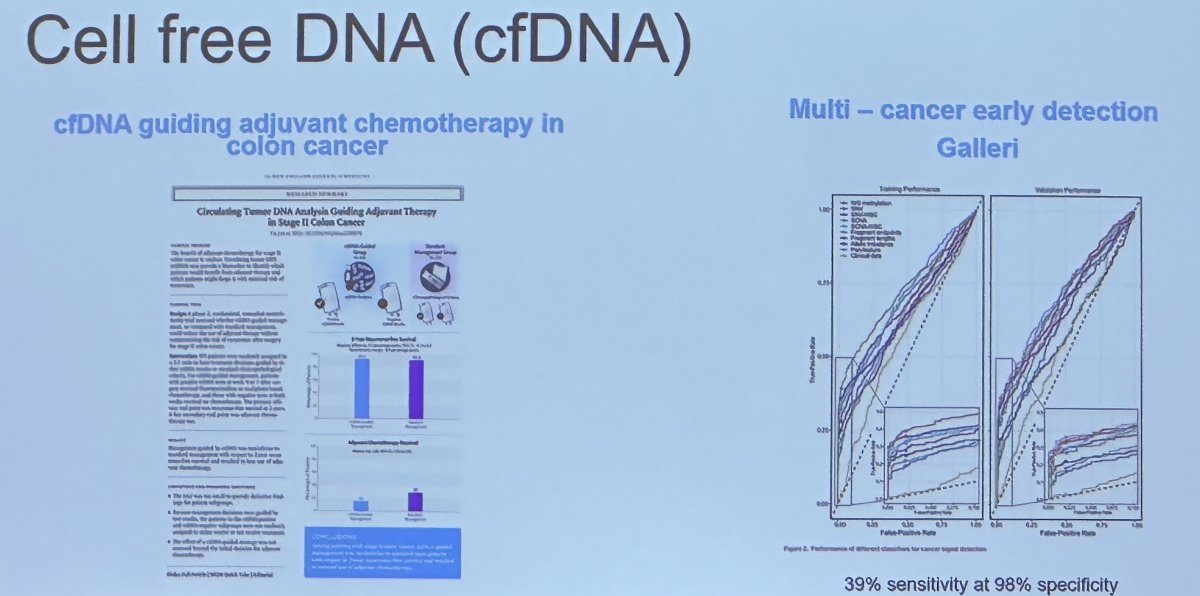
Circulating tumor cells may be of benefit in the metastatic disease states, whereby genomic mutations may be predictive of treatment response. For example, the ARV7 mutational status has been shown to predict response to enzalutamide/abiraterone versus taxanes. The role of cell free DNA (cfDNA) is emerging in other oncologic disease sites, and Dr. Carroll noted that he expects this to become relevant to the prostate cancer disease space in the upcoming few years.
Dr. Carroll concluded that somatic testing:
- May complement good clinical testing
- May be useful in certain clinical states
- Use of serum tests is rapidly evolving with
- cfDNA
- CTCs
Presented by: Peter Carroll, MD, MPH, Professor, Department of Urology, University of California in San Francisco, San Francisco, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:- Spohn SKB et al. Int J Radiat Oncol Biol Phys, 2022.
- Klein EA et al. A 17-Gene Assay to Predict Prostate Cancer Aggressiveness in the Context of Gleason Grade Heterogeneity, Tumor Multifocality, and Biopsy Undersampling. Eur Urol, 2014.
- Tward J et al. The Clinical Cell-Cycle Risk (CCR) Score Is Associated With Metastasis After Radiation Therapy and Provides Guidance on When to Forgo Combined Androgen Deprivation Therapy With Dose-Escalated Radiation. Int J Radiat Oncol Biol Phys, 2022.
- Fizazi K et al. Rucaparib or Physician's Choice in Metastatic Prostate Cancer. N Engl J Med, 2023.


