(UroToday.com) Dr. Neal Shore continued the morning plenary session with an excellent presentation on the late breaking abstract regarding the EMBARK clinical trial for high risk biochemically recurrent prostate cancer. The trial started recruitment in January 2015 and accrued the final patients in August 2018.
Dr. Shore introduced the rationale for the trial design by explaining that up to 20-50% of patients will experience a biochemical recurrence after definitive therapy for prostate cancer as evidenced by a rising PSA. Patients with high-risk biochemical recurrent prostate cancer, i.e. rapidly rising PSA with decreased doubling time are known to have a higher risk of mortality and progression of disease. Limited level 1 evidence exists as to how best to treat these patients. Recent phase 3 clinical trials have shown some benefit to aggressive therapy with novel androgen receptor signaling blockers in these high risk patients. As such the EMBARK trial sought to evaluate Enzalutimide in combination with Leuprolide compared to Leuprolide plus placebo and Enzalutimide alone in high risk biochemically recurrent prostate cancer patients.
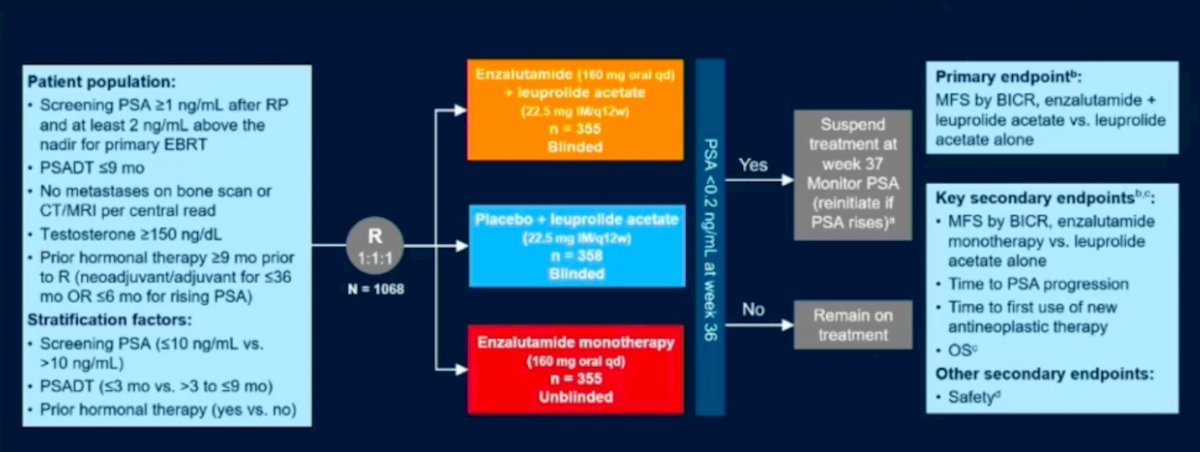
Patients included in the trial included patients with PSA > 1 ng/mL after radical prostatectomy or PSA > 2 ng/mL above the nadir following radiation therapy, with a PSA doubling time of < 9 months constituting the high risk BCR group. All patients had to be clear of metastatic disease on conventional imaging and non-castrate levels of testosterone. The patients were sub stratified into very high and high risk groups based on PSA > 10 ng/mL vs < 10 ng/mL and PSA doubling time of < 3 months or > 3 months – 9 months. A total of 1058 patients were randomized in a ratio of 1:1:1 to receive either Enzalutimide in combination with Leuprolide compared to Leuprolide plus placebo or Enzalutimide alone. At 36 weeks patients were evaluated for PSA response and if < 0.2 ng/mL were placed on a treatment holiday until PSA levels rose or if > 0.2 ng/mL then patients were continued on treatment. The primary endpoint was a composite metastasis free survival (MFS) for Enzalutimide + Leuprolide vs Leuprolide alone. Secondary endpoints included MFS for Enzalutimide alone vs Enzalutimide + Leuprolide, time to PSA progression, overall survival, and the first use of other antineoplastic agents. Safety profiles of the various arms were also evaluated.
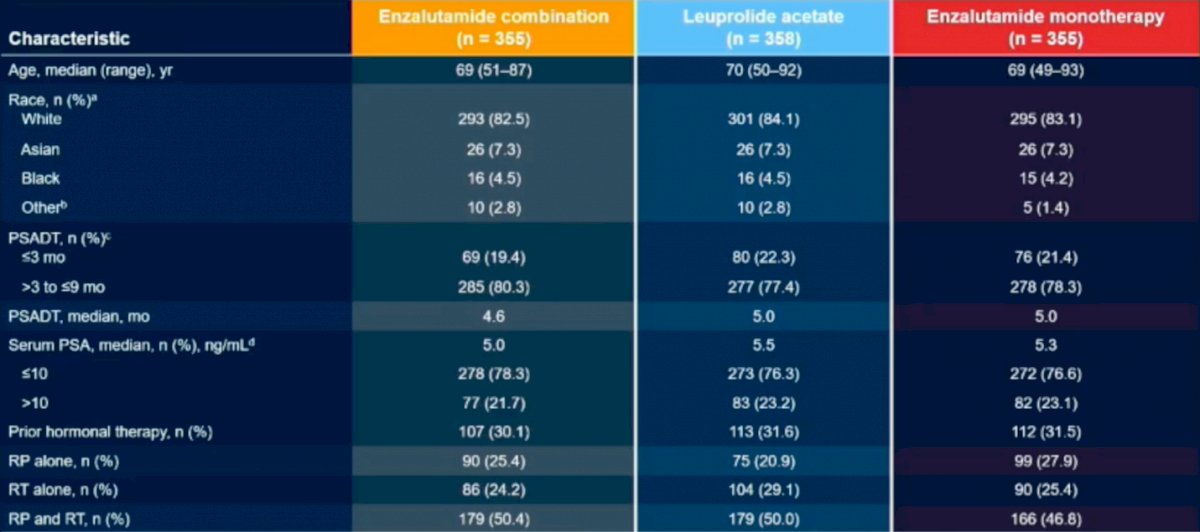
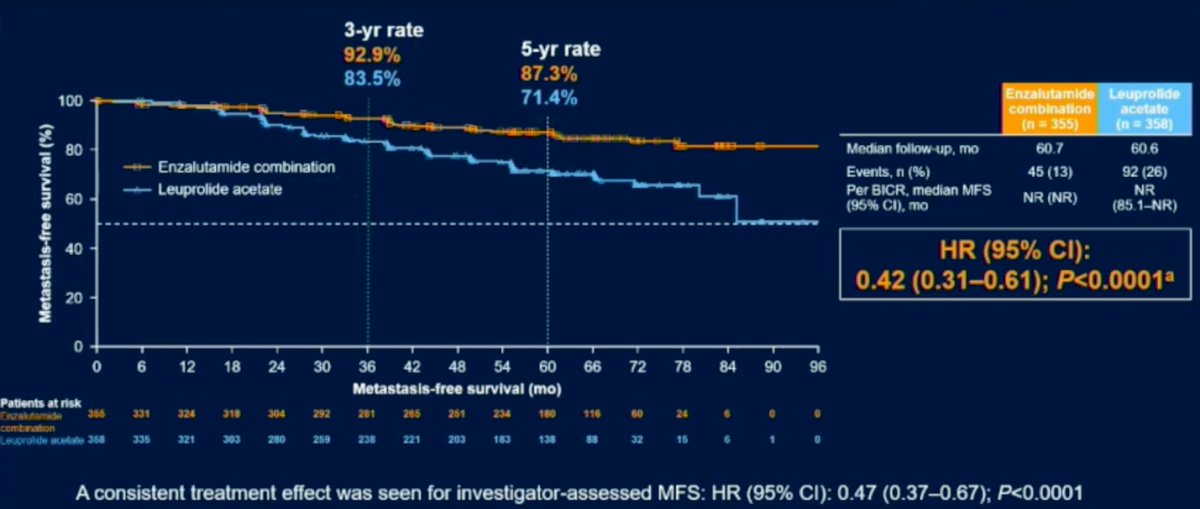
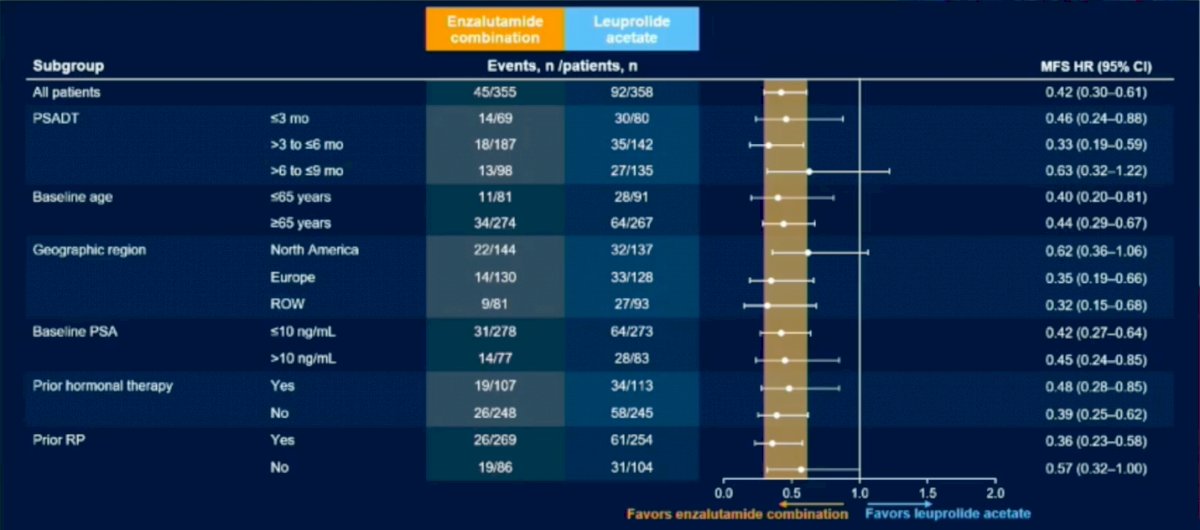
Patient demographics were similar amongst all groups. The results revealed a 58% reduction in progression or death for patients on Enzalutimide plus Leuprolide vs Enzalutimide alone (HR (95% CI): 0.42 (0.31-0.61); P<0.0001). Subgroup analysis revealed that regardless of PSA doubling time, age, geographic region, baseline PSA or prior hormonal therapy all patients fared better in the Enzalutimide plus Leuprolide arms compared to Leuprolide alone.
The interim overall survival analysis revealed a trend toward significance in the Enzalutimide plus Leuprolide group vs Leuprolide alone (HR (95% CI): 0.59 (0.38-0.90) P=0.0142) due to the preset boundary for significance at <0.001.

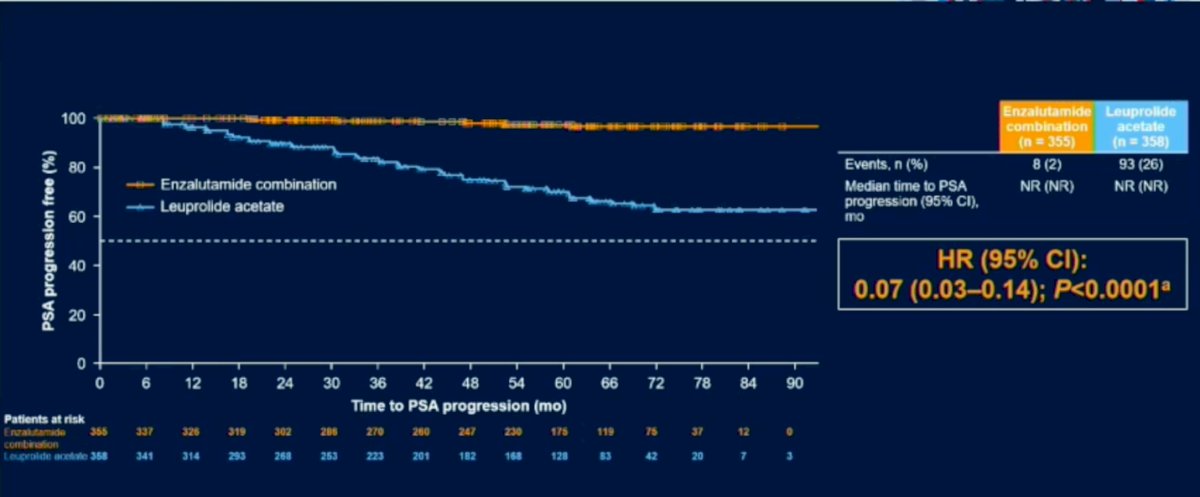
There was a significant difference in time to PSA progression with minimal progression events in the Enzalutimide plus Leuprolide arm (HR (95% CI): 0.07 (0.03-0.14); P<0.0001). Similarly, benefit was seen in Enzalutimide plus Leuprolide arm in the use of other antineoplastic agents (HR (95% CI): 0.36 (0.26-0.49); P<0.0001). With regards to MFS in the Enzalutimide alone arm versus Leuprolide monotherapy benefit was seen in the Enzalutimide group (HR (95% CI): 0.63 (0.46-0.87); P=0.0049).
91% of patients in the Enzalutimide combination group experienced PSA response to < 0.2 ng/mL with median duration to treatment holiday being 20.2 weeks compared to 67% and 16.8 weeks in the Leuprolide monotherapy group and 85.9% and 11.1 weeks in Enzalutimide alone.

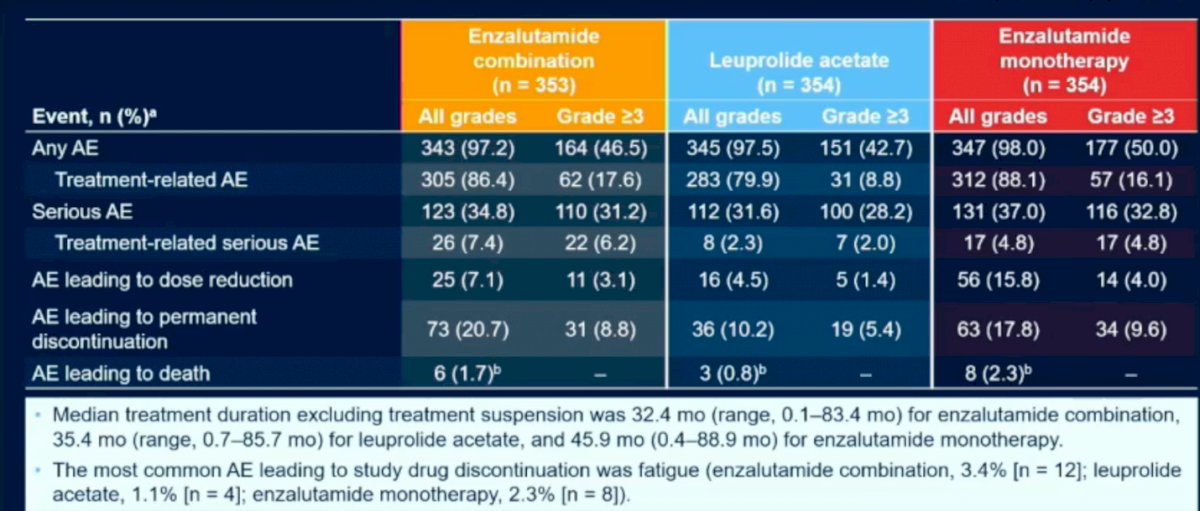
With regard to the safety profile of the various treatment arms, there were no significant differences between the groups. The most common cause of discontinuation in either treatment arms was fatigue. There were no adverse events leading to death in either of the treatment arms.
Dr. Shores concludes that compared to Leuprolide monotherapy, Enzalutimide in combination with Leuprolide provides a statistically significant and clinically meaningful improvement in MFS in all high risk BCR recurrence patients. There are significant delays in time to PSA progression and the need for further antineoplastic treatment. Finally, there is a trend towards improvement in overall survival on interim analysis in the Enzalutimide combination group.
Presented by: Neal D. Shore, MD, FACS, Medical Director, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Sohrab Naushad Ali, MD, MSc, FRCSC, Assistant Clinical Professor, Department of Urology, University of California Irvine, @sohrabnaushad on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023


