(UroToday.com) Differential renal function remains a crucial indicator for the management of certain kidney disorders. As of recent, nuclear medical imaging serves as a common modality to offer urologists a view of the renal unit as well as an estimate of kidney function. During the 2023 AUA Meeting, Dr. Daniel Salevitz, from the Mayo Clinic in Arizona, delivered an presentation regarding the use of an artificial intelligence model to evaluate differential kidney function. Specifically, his team sought to do so with automated methods of assessing contrast enhanced computed tomography in tandem with machine and deep learning algorithms, without the use of nuclear medical imaging.
Dr. Salevitz and his colleagues developed a deep learning model for the purposes of automatic segmentation. To start, segmentation trained the algorithm to recognize specific areas of interest.
Following, the applications of radiomics allowed for the extraction of features used to compare both kidneys and train the algorithm. 2D and 3D radiomics features were examined and extracted in order to compute delta radiomics and determine the algorithm’s ability to approximate kidney function. Performance was evaluated with AUC, precision, recall, F1 score, and receiver operating characteristics. Moreover, dice scores were determined as a function of the model’s accuracy. Patients who received renal nuclear medical scanning within the years 2018-2022, at Mayo Clinic, were collected to be a part of this study (n=4116). From this group, kidneys with computerized tomographic (CT) scans, taken within a three-month period from the nuclear scans were precluded. Three hundred thirty-three CT scans were chosen, at random, to be reviewed. Additionally, any patient that obtained urological or radiological care during the given time frame was excluded.
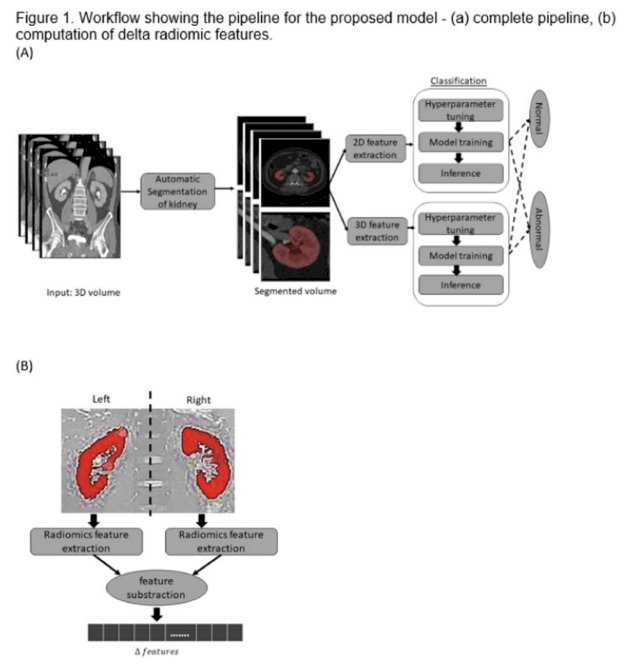
As seen above, this figure is a visual representation of the workflow implemented in order to develop the fine-tuned model.
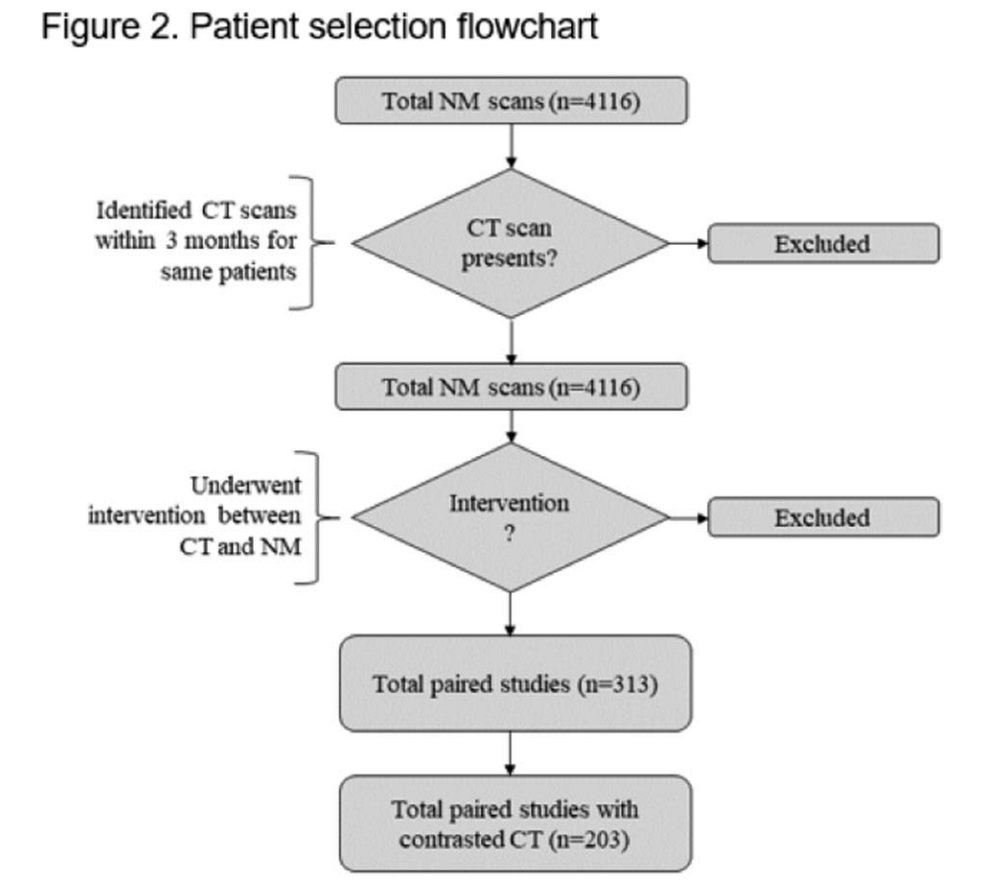
This figure (above) depicts the process of patient selection for this study.
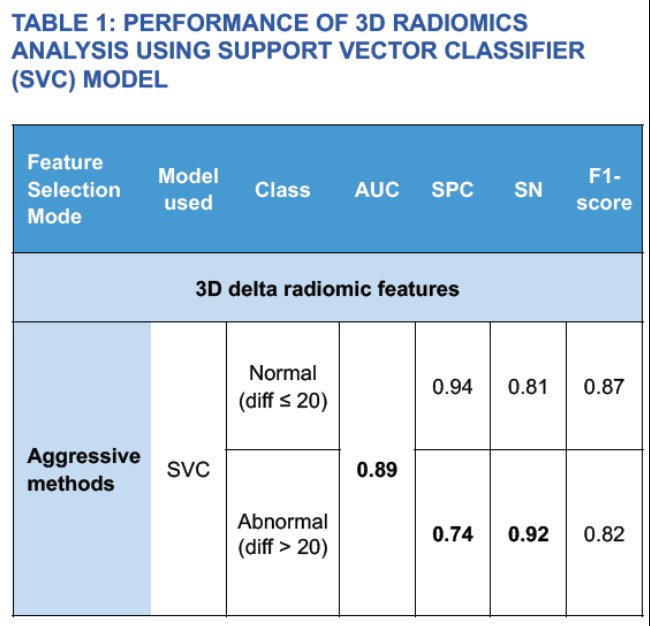
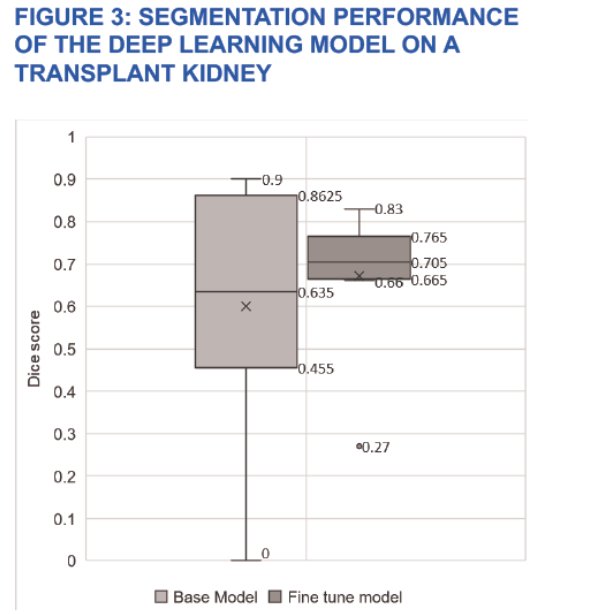
Notably, Dr. Salevitz and his team found promising results with the use of their automated model. The segmentation model exhibited a Sorenson-Dice score of 0.7. Consistently, 3D radiomics outperformed 2D analysis. Interestingly, they found the best outcomes on 3D radiomics with the application of a Support Vector Classifier (SVC) model coupled with aggressive feature selector schema, achieving 92% sensitivity and 74% specificity. While the model displayed poor segmentation on structurally abnormal kidneys, good segmentation was seen for transplant kidneys (Figure 3). Furthermore, kidney number, location, presence of anatomical abnormalities, such as cysts and masses, and presence of foreign bodies had no effect on the model’s segmentation. As seen in Table 1 (below), this AI model achieved an AUC score of 0.89 with 0.80 and 0.80 precision and recall, respectively. The F1 score was 0.82. Dr. Salevitz and his team concluded that machine learning has the capability to detect subtle features in kidneys that can be used to estimate a numerical value of kidney function.
At the end of his presentation, Dr. Salevitz was asked a few questions involving the clinical applications of this algorithm. When asked about the benefit of this model for future patients, he emphasized the decrease in cost and increased accessibility of CT scans when compared to nuclear medical imaging. Though it has not yet been established into clinical practice, this artificial intelligence model shows great promise in the identification and management of kidney diseases (kidney cancer, obstructions, etc.) as well as urological surgical planning in the future.
Presented by: Daniel Salevitz, MD, Mayo Clinic Arizona, Phoenix, AZ
Written by: Candices Tran, B.S., University of California, Irvine, @candicesmtran on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023


