One of the key issues in the management of patients with low-grade UTUC is the over-reliance on radical nephroureterectomies. In an NCDB analysis of 12,160 patients with low-grade UTUC tumors, 52% of such patients were treated with RNU.1 This clearly represents over-treatment for the majority of patients with low-grade UTUC. Treatment with RNU may also have critical consequences, which includes progression to renal insufficiency post-RNU, with nearly 80% of patients with UTUC having stages 3-5 chronic kidney disease post-surgery.

This decrease in renal function may limit the possibility of administering adjuvant cisplatin-based chemotherapy regimens and would render patients with contralateral disease undergoing an RNU dialysis/transplant dependent.

In lieu of an RNU for such patients, endoscopic-only management of such patients is associated with a recurrence rate of approximately 65%. This is further complicated by the fact that such patients have to undergo numerous such endoscopic procedures, with a retrospective analysis of 1,027 patients demonstrating that ~17% underwent ≥2 endoscopic procedures in the year after a UTUC diagnosis. Furthermore, patients progressed to RNU <1 year after endoscopic procedures with a median time of ~36 days.

As such, JELMYTO has emerged as an alternative/complementary treatment modality for the management of such patients. JELMYTO allows for the sustained exposure of the collecting system/ureter to mitomycin making effective chemoablation of low-grade tumors possible. Due to the RTGel® reverse-thermal hydrogel technology, JELMYTO allows for a dwell time of 4-6 hours. JELMYTO is instilled as a chilled liquid, via a ureteral catheter or nephrostomy tube, which fills and conforms to the renal pelvis, and then subsequently changes into a semisolid gel at body temperatures for 4-6 hours.

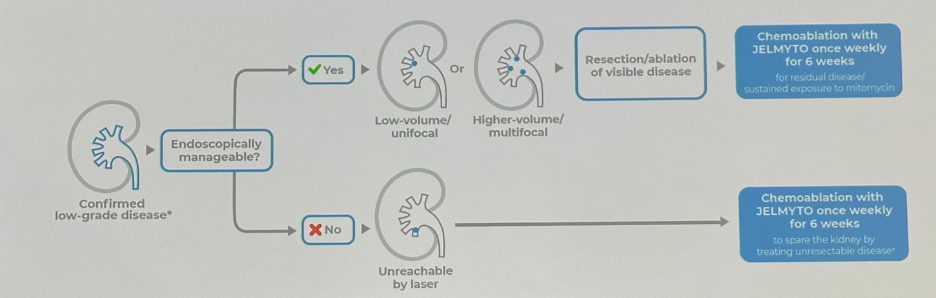
JELMYTO can be administered alone or subsequent to endoscopic management as demonstrated in the figure below. It is important to note that JELMYTO should not be instilled immediately following resection or ablation.
JEMLYTO was evaluated in the pivotal phase 3 trial, OLYMPUS, which subsequently led to the FDA approval of this drug. The OLYMPUS trial was a phase 3, open-label, single arm, multicenter prospective study that evaluated six weekly induction course of JELMYTO in patients with new or recurrent non-invasive LG UTUC. The primary endpoint was complete response (CR) and patients who achieved this outcome went on to a once monthly maintenance dose of JELMYTO for up to 12 months. 12-month durability of CR was a significant secondary endpoint.
- All patients had biopsy confirmed LG UTUC with cytology negative for high grade disease.
- At least one papillary low-grade tumor located superior to the ureteropelvic junction had to be present
- Size: 5-15 mm
- Patients who had larger tumors could have had tumor debulking prior to treatment in order to meet the criteria
- The primary endpoint of CR was defined as:
- Complete absence of tumor lesions in the ipsilateral pyelocaliceal system at 3 months after initiation of JELMYTO
- Evaluated via urine cytology, ureteroscopy, and biopsy, where warranted
- The safety objective was to evaluate the safety and tolerability of JELMYTO in patients with low-grade UTUC
- The trial allowed for the instillation of JELMYTO into the pyelocaliceal system via ureteral catheters or nephrostomy tubes

The baseline patient characteristics are summarized in the table below. It’s important to note that pre-debulking, the mean number of lesions was 2.2 and after debulking the median was 1 lesion. The mean diameter of the largest tumor was 8 mm. Among the 71 patients, 11% were uninephric, 52% had prior history of low-grade UTUC, and almost 50% had unresectable tumors.

A complete response rate was achieved in 58% of patients. A further 11% achieved a partial response, with 17% having no response.
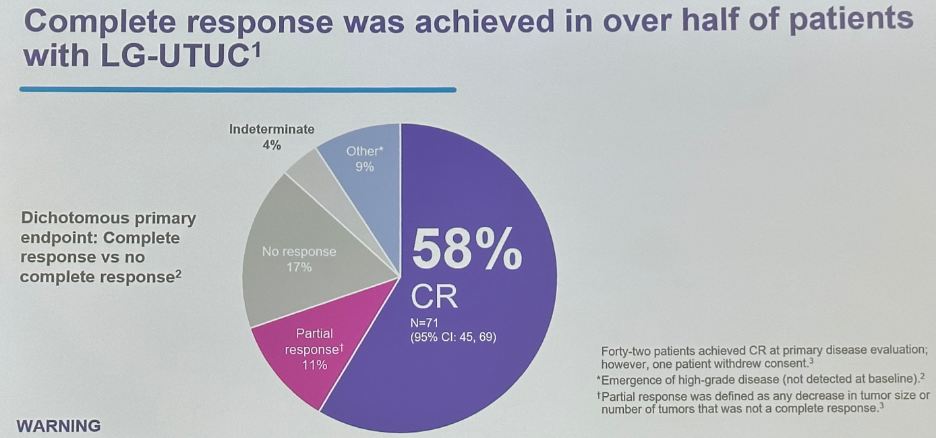
As highlighted above, nearly half of the patients in the OLYMPUS trial had endoscopically unresectable tumors, and the rate of complete responses was similar among patients in this subgroup.

Significantly, JELMYTO demonstrates durability of response in patients achieving a complete response for up to 12 months. Based on the OLYMPUS trial, 82% of patients with a complete response maintained such a response for 12 months. At the 12-month assessment of durability in the 41 evaluable patients, 56% maintained a complete response, with a median duration of response not yet reached (range: 0 – 18.8 months).

Interim results from the OLYMPUS long-term follow-up study demonstrated in a subset of 16 patients who had previously achieved a complete response at the OLYMPUS 12-month assessment:
- 29 months median duration of response (range: 14.6 – 47.6 months)
- 13 (81%) patients with continued complete responses beyond 12 months after primary disease evaluation
- 2 patients had low-grade UTUC recurrence
- 1 patient had RNU following ureteral stricture
The adverse event profile of JELMYTO is summarized below:

We highlight the often-discussed complication of ureteral obstruction post-JELMYTO. Any grade ureteric obstruction occurred in 58% of patients, with grade 3 or worse occurring in 17%. It is important to note that this definition of ureteric obstruction was quite broad and included events such as development of interval mild hydronephrosis. Furthermore, it remains currently unclear what proportion of such ureteric obstruction rates are secondary to JELMYTO itself versus the retrograde instrumentation for all patients in the OLYMPUS trail. As such, many have advocated for the use of antegrade instillation via a percutaneous nephrostomy tube. It is important to highlight that JELMYTO offers a choice of administration with the ability to instill at multiple sites:

Drs. Linehan and Prasad next went on to highlight important safety information regarding JELMYTO, including:
- Contraindications for patients with perforation of the bladder or upper urinary tract
- Ureteric obstruction as discussed above
- Bone marrow suppression secondary to JELMYTO. As such, a complete blood count with a differential is recommended prior to each treatment.
- Potential embryo-fetal toxicity
- In 2-10% of patients: Urinary tract inflammation, bladder spasms, urosepsis, hypersensitivity, and instillation site pain
Next, Drs. Linehan and Prasad went on to highlight a multicenter real-world study of JELMYTO use for patients with UTUC.3 This study by Woldu et al was the largest retrospective report of patients treated with JELMYTO at 15 academic institutions and community centers. The median follow-up was 6.7 months following JELMYTO induction (IQR: 3.5 – 11.3 months). JELMYTO was used in the primary chemoablative setting and following varying degrees of endoscopic management. 136 renal units from 132 patients were treated/included. The majority of cases were biopsy proven low-grade UTUC.
The median patient age was 74 years, with 22% of such patients having a solitary kidney (11% in OLYMPUS). 88% of patients had low-grade disease, with the remaining not biopsied or grade being unknown. 75% had no prior history of UTUC. The tumor was completely ablated in 43% of patients, with 15% having >3 cm residual tumor. Tumors were unifocal in 51%, with 18% having >3 tumors. Most tumors were located above the UPJ.

As demonstrated in the figure below, 43% of patients had JELMYTO administered antegrade via a percutaneous nephrostomy (all patients in OLYMPUS had retrograde instillations). The remainder had retrograde instillations in clinic (36%) or in a hospital setting under anesthesia (21%). The use of maintenance doses of JELMYTO was reported in 27% of cases (n=16).

The efficacy of JELMYTO varied with the tumor size prior to induction. A subgroup analysis of patients with biopsy proven low-grade UTUC (n=100) demonstrated:
- No visible tumor prior to induction: 75% with no evidence of disease after JELMYTO induction
- <1 cm tumor: 78% with no evidence of disease
- 1 – 3 cm tumor: 35% with no evidence of disease
- >3 cm tumor: 25% with no evidence of disease
With regards to adverse events, 23% of patients had clinically significant ureteral stenosis, defined as ureteral obstruction that required a ureteral stent or nephrostomy tube or that would typically require a ureteral stent or nephrostomy tube. This rate was significantly lower than that observed in the OLYMPUS trial (44%), which may reflect differences in the definition of ureteral obstruction and potentially a lower rate of stenosis with the antegrade instillation (43% in this cohort) as opposed to retrograde instillation (100% in OLYMPUS).

The limitations of this study are summarized in the illustration below:

Drs. Linehan and Prasad concluded their presentation by highlighting the available UroGen SupportTM offerings:
Presented by:
- Jennifer A. Linehan, MD, Associate Professor, Urology and Urologic Oncology, John Wayne Cancer Institute, Santa Monica, CA
- Sandip M. Prasad, MD, MPHil, Atlantic Health System, Morristown, NJ
the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
References:1. Upfill-Brown A, et al. Treatment utilization and overall survival in patients receiving radical nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma: evaluation of updated treatment guidelines. World J Urol, 2019.
2. Petros FG, et al. Endoscopic Approaches to Upper Tract Urothelial Carcinoma. Urol Clin North Am, 2018.
3. Woldu SL, et al. Early experience with UGN-101 for the treatment of upper tract urothelial carcinoma – A multicenter evaluation of practice patterns and outcocmes.


